- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.14
Aim of investigation is to reproduce the main artery restenosis on before implanted intravascular stent in experiment.
Materials and Methods: As the prototype of main artery in experiment, we use an original device for modeling intra-arterial circulation (document of invention RU 202780). The main part of it is a glass tube rotameter connected with two silicone tubes. The pump makes the imitation of arterial blood circulation. We use an aqueous solution of glycerol, diluted corresponding to the viscosity of human blood. Through the fitting we introduce into rotameter glass tube a dye (ink), a silk thread and pressure probe.
Results: We use a metal device 71 mm length, made in the form of a grid with beams directed in a spiral as a prototype of intravascular stent. We observed an increasing of the pressure level up by 56% during the spreading of the wave after the pause between cardiac cycles for 1,5 seconds compared with the regular heart rhythm. We observed reflected, standing waves in the glass tube of rotameter.
Conclusion: The pulse wave after the pause between cardiac cycles for 1,5 seconds and more is associated with the term of “hydraulic shock” in the arterial vessel. The mechanical effect of it has the great importance on the process of restenosis if there’s primary installed intravascular stent.
Keywords: modeling of intra-arterial circulation, experimental cardiology, device for modeling, restenosis.
In interventional cardiology and vascular surgery, remains the problem of restenosis on before implanted stent in coronary arteries, as well as in carotid arteries. Prevention measures are associated with studying and correction of the restenosis risk factors, improving the stent characteristics, following all the necessary drug treatment [1-5]. But, unfortunately, we’re still far from solving this problem and the patient appears to have the signs of restenosis in different arterial areas, achieving in some cases 40% [6-9]. The main mechanism for the development of restenosis is considered proliferation of neointima at the site of stent placement [10-12]. The main risk factors and pathophysiological mechanisms are female sex, diabetes mellitus, small initial minimum vessel diameter, short lesions, associated with intima inflammation with further proliferation [13-19]. In our previous clinical research, we revealed more frequent restenosis in patients with arrhythmias [20, 21].
Aim of investigation is to reproduce the main artery restenosis on before implanted intravascular stent in experiment.
We used the original device for modeling of intra-arterial circulation created by collaborative work of cardiologists and engineers (document of invention RU 202780). To reproduce main arterial vessel, the main part of the device was made of glass tube rotameter with an inlet diameter of 20 mm, outlet diameter 16,5 mm and length 365 mm, with the thickness on the glass tube is 2 mm. The rotameter is fixed with four steel rods and couplings to a horizontal table. Free ends of the glass tube are connected with two silicone tubes, with other ends connected to the pump, powered by a 12 Volt battery. We inject the fluid into the closed system. As such a liquid, we use an aqueous solution of glycerin diluted corresponding to the viscosity of human blood. Being transparent, it allows observing the processes inside the tube and performing the necessary measurements. A fitting is located at the inlet end of the tube, through which we can introduce a dye (ink) inside the tube, as well as silk thread of contemporary length, and intravascular pressure probe. The main part of the device is presented on figure 1. The pump is able to make the circulation of liquid in closed circuit in the pulse-wave mode, thus simulating pulse waves in regular heart rhythm, as well as in atrial fibrillation. When simulating coronary blood flow, we took into account its dual nature both in systole and in diastole of the heart.
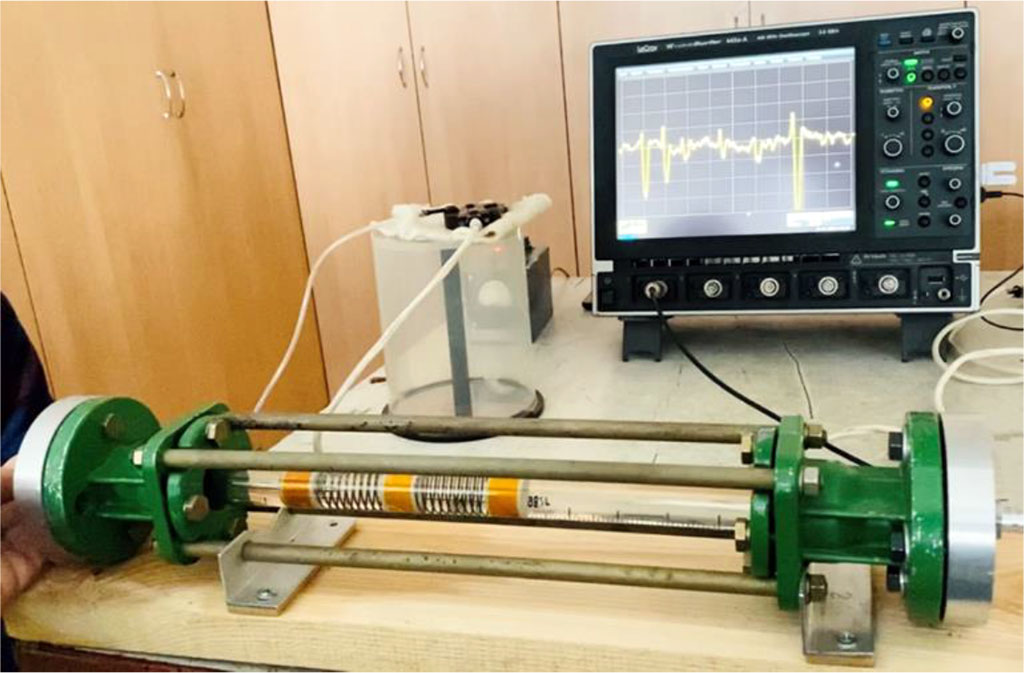
Figure 1. Device for modeling of intra-arterial circulation (general view).
As an intravascular endoprosthesis, we used a special device 71 mm length and 18 mm diameter, made in the form of a grid with beams directed in a spiral (beam thickness 0,8–1 mm). The endoprosthesis was inserted from the input end of the glass tube to tight contact with the inner surface walls. It was fixed inside the tube and did not move with the fluid flow. Next, we installed a conductor with a 5 cm long silk thread inside the endoprosthesis. After that, we put the pump to work both with the regular heart rhythm and atrial fibrillation with different pauses between pulse waves. After the long pauses between cardiac cycles in atrial fibrillation, we observed appearance of reflected, standing waves on the intravascular stent. In case of atrial fibrillation, during the spread of the wave after the long pauses for 1,5 seconds and more, we observed an intense effect of the wave pressure (the indicator was a silk thread) on the walls of the rotameter tube, with the formation of reflected, standing waves, with significant displacement of the thread in perpendicular direction.
When a piezocrystalline pressure sensor was placed inside the tube, the measurements confirmed our visual observations. Thus, the increase in pressure in atrial fibrillation during the spread of the wave after the long pauses for 1,5 seconds and more with repeated measurements had level up by 56% compared with the pressure in simulation the regular heart rhythm.
We inserted plastic diaphragm into the tube with a narrowing of the inner hole, simulating the stenosis of 70% of the inner diameter of the tube. By analogy with the first part of the experiment, we observed the movement of the silk thread that moved relative to the diaphragm to different depths. During the pulse waves after the long pause for 1,5 seconds and more in atrial fibrillation imitation, the intense impact of reflected and standing waves on the edge areas of the diaphragm was observed, as well as intra-arterial pressure level up by 56% in comparison with the regular heart rhythm waves (Figures 2, 3).

Figure 2. Intravascular stent modeling (endoprosthesis, diaphragm and silk thread are installed inside the rotameter tube). Reflected, standing waves in atrial fibrillation imitation after the pauses for 1,5 seconds and more – the indicator – thread – in different periods of time.
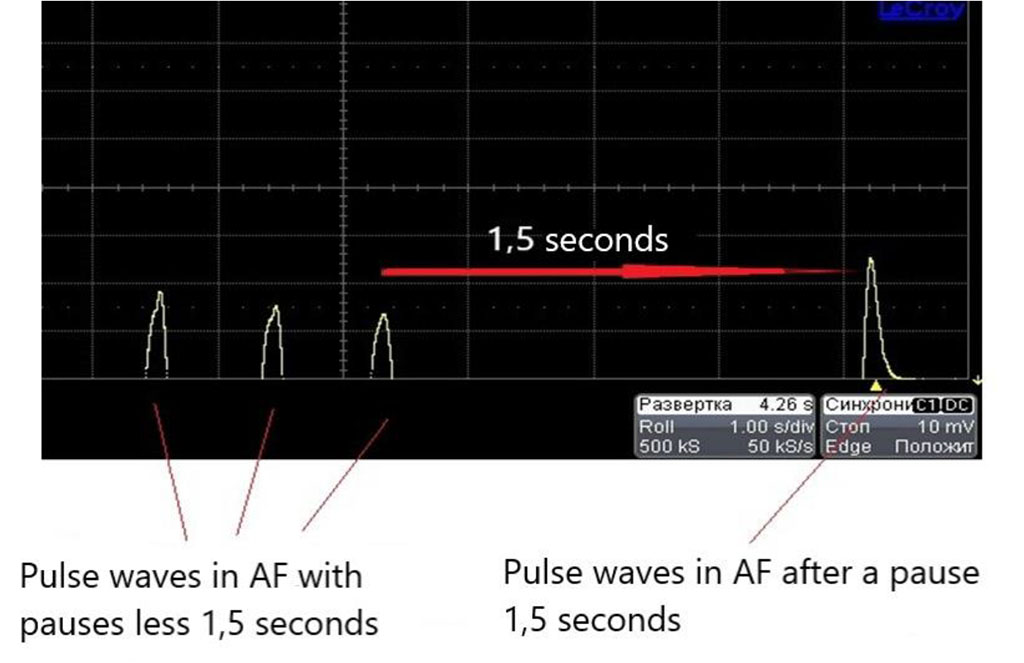
Figure 3. Data from oscilloscope connected with piezoelectric pressure probe in atrial fibrillation simulation (the biggest wave is registered after the pause for 1,5 seconds and more).
Using the device for modeling of intra-arterial circulation, we were able to visualize, quantitatively and qualitatively characterize the hemodynamic processes inside the arterial vessel in regular heart rhythm and atrial fibrillation. First, we were interested in whether the appearance of additional factors of mechanical action on the arterial vascular wall occurs during the spread of the wave after the long pauses between ventricular contractions in atrial fibrillation.
In our previous publications, we wrote about the increase in the main factors of the kinetics of the main arteries during the heart arrhythmias [20-23]. So, we used the experimental modeling to confirm the clinical results.
In this experimental work, we obtained an increase in intravascular pressure, measured by piezocrystalline pressure sensor, during the spread of the wave after a long pause between ventricular contractions in atrial fibrillation, with level up to 56%, compared with the regular heart rhythm wave. We observed the occurrence of standing and reflected pressure waves inside the rotameter tube. Especially the mechanical impact of pressure waves was intense on the edge areas of the diaphragm. We can characterize this phenomenon by the concept of "hydraulic shock". This term means an additional mechanical effect of increased hemodynamic parameters of intravascular pressure on the wall of the arterial vessel; it occurs during the spread of the first wave after a long pause between ventricular contractions in atrial fibrillation.
Thus, in an arterial vessel with atrial fibrillation, especially bradysystolic, an increase in hemodynamic parameters occurs with a predominant mechanical effect on the marginal zones of an existing plaque. If a stent is installed inside the coronary artery, then the impact of a mechanical pressure wave of the first wave after a long pause between ventricular contraction can be the very traumatic factor that initiates and stimulates the growth of the neointima, triggers a series of pathophysiological processes leading to the development of neoatherosclerosis and restenosis on the implanted intravascular stent. This statement is true for the coronary arteries and for the renal arteries, as well as for the carotid arteries, where intravascular stents can be installed.
With the spread of the wave after a long pause for 1,5 seconds and more in atrial fibrillation, a hydraulic shock occurs in the arterial vessel. The mechanical effect of hydraulic shock can be the starting point for the onset and progression of restenosis in an implanted intravascular stent. Timely treatment and correction the heart rate in atrial fibrillation, with avoiding the long pauses between ventricular contractions can be very helpful in prevention of restenosis.
The capabilities of the device for modeling of intra-arterial circulation make it possible to perform the experimental part of a wide range of work in cardiology, cardiovascular surgery, normal and pathological physiology.