- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 4. DOI 10.35630/2024/14/4.404
Sleeve gastrectomy has now become one of the most popular operations in the treatment of morbid obesity. We present a clinical case of minimally invasive treatment of early suture failure after sleeve gastrectomy, from the archive of the authors’ clinical observations. Suture failure occurred on the day 4 after surgery. Endogastroscopy revealed a defect of 12 mm in diameter with infiltration at the edges. The technique of endoscopic sanitation of the abscess cavity and transcutaneous drainage of the abdominal cavity was used. After 14 days, the drainage in the abdominal cavity stopped functioning. Fistulography showed that it was not in the abscess cavity; according to endoscopic fistulography, hermetic sealing was achieved. After 2 months the fistula was completely closed. The patient was discharged with complete recovery. After 6 months, EGD revealed cicatrical changes; body weight loss: 55 kg.
This clinical case demonstrated the possibility of combined minimally invasive treatment of a postoperative complication – gastric suture failure after sleeve gastrectomy.
Keywords: sleeve gastrectomy, postoperative complications, suture failure, morbid obesity, treatment of complications
Recently, due to unhealthy diet and lifestyle, the number of patients with morbid obesity has increased significantly. The number of surgical interventions for obesity in the world has increased fivefold over 15 years (140,000 in 2003 versus 720,000 in 2018) [1, 2].
When it comes to different types of surgeries, there is a great variation in the techniques performed. Thus, in the early 2000s, Roux-en-Y gastric bypass and adjustable gastric banding constituted the vast majority of bariatric procedures (about 90%), while sleeve gastrectomy was still in its infancy [3, 4]. Vertical gastrectomy is a bariatric operation aimed at reducing the volume of food intake without disturbing the physiological passage through the gastrointestinal tract and consists in removing approximately 3/4 of the stomach volume at the expense of the anatomical fundus and greater curvature [5, 6]. Very often this operation is called an English term “sleeve gastrectomy”. The following terms are found in the literature: “vertical gastrectomy”, “curvature gastrectomy”, “parietal gastrectomy”. The Russian-language literature includes the terms “sleeve gastroplasty”, “sleeve gastrectomy” and “vertical gastrectomy”. Although, in essence, such an operation is not a gastrectomy, but a stomach resection.
There are a number of controversial issues regarding the technique of this operation. Some researchers believe that after mechanical resection the mechanical suture line must be buried using a continuous suture. Others demonstrate that there is no clear data confirming the effectiveness of that technique and describe that an additional suture may increase ischemia and the number of leaks. Along with excess weight loss, important indicators of the effectiveness of surgical procedures are the dynamics of concomitant diseases and the quality of patients’ life after surgery [7].
In the treatment of obesity, one should follow a comprehensive, integrated approach. However, it is known that today, bariatric surgery is the most effective method of treating obesity [8-11].
Unfortunately, like any technique, sleeve gastrectomy has its complications. Currently, 30-day morbidity and mortality rates after laparoscopic sleeve gastrectomy, according to the literature, range from 0 to 17.5% and from 0 to 1.2%, respectively. The most common complications are bleeding, suture failure, stricture, and nutritional deficiency [12-13]. The incidence of postoperative suture failure after laparoscopic sleeve gastrectomy ranges from 2 to 3% [14, 15]. The cause of suture failure in patients after sleeve gastrectomy is the increased pressure on the staple line under conditions of relative ischemia [16, 17]. It is usually located just below the gastroesophageal junction, where there is relative ischemia due to the short gastric vessels transected during the surgery. Increased pressure may also be due to distal narrowing caused by a small size of the tube, a stricture, or a technical error associated with running the stapler too close to the gastric angle.
There have been mixed results and opinions regarding whether peritonization of staples can reduce the incidence of suture failure, and further research is needed on this issue. Larger bougies have been demonstrated to reduce the incidence of suture failure in sleeve gastrectomy patients. Currently, there is also no consensus regarding the methods of treatment tactics in the event of postoperative complications [18-20].
The purpose of our work is to present a clinical case of minimally invasive treatment of early suture failure after sleeve gastrectomy from the authors’ archive of clinical observations.
Female patient P., 54 years old, was admitted to the clinic with a diagnosis of stage III obesity, metabolic diabetes mellitus, symptomatic hypertension. On admission, body weight was 165 kg, height 162 cm, BMI 62.87.
After further examination, trocars of 4 – 5 mm, 1 – 10 mm and, during the second stage, 1 – 13 mm were installed through classical points for insertion of a stapler. The greater curvature was mobilized using “LigaSure” (Coviden, USA). Next, with the help of a Panther stapling device, a drainage-resection of the stomach was performed (7 cassettes were required). A hydrotest was carried out using a dye, the hermetic sealing of the suture line was achieved. A nasobiliary tube was installed, as well as a drainage into the abdominal cavity (subhepatic space).
The postoperative period was smooth, however, on the day 4, epigastric discomfort appeared and the temperature increased to 38°C. It should be noted that in patients with severe obesity, the classic clinical picture of peritonitis is not seen and, as a rule, there is no inflammatory reaction in the peripheral blood. EGD revealed a defect of 12 mm in diameter with infiltration at the edges in the prepyloric region. Fig. 1
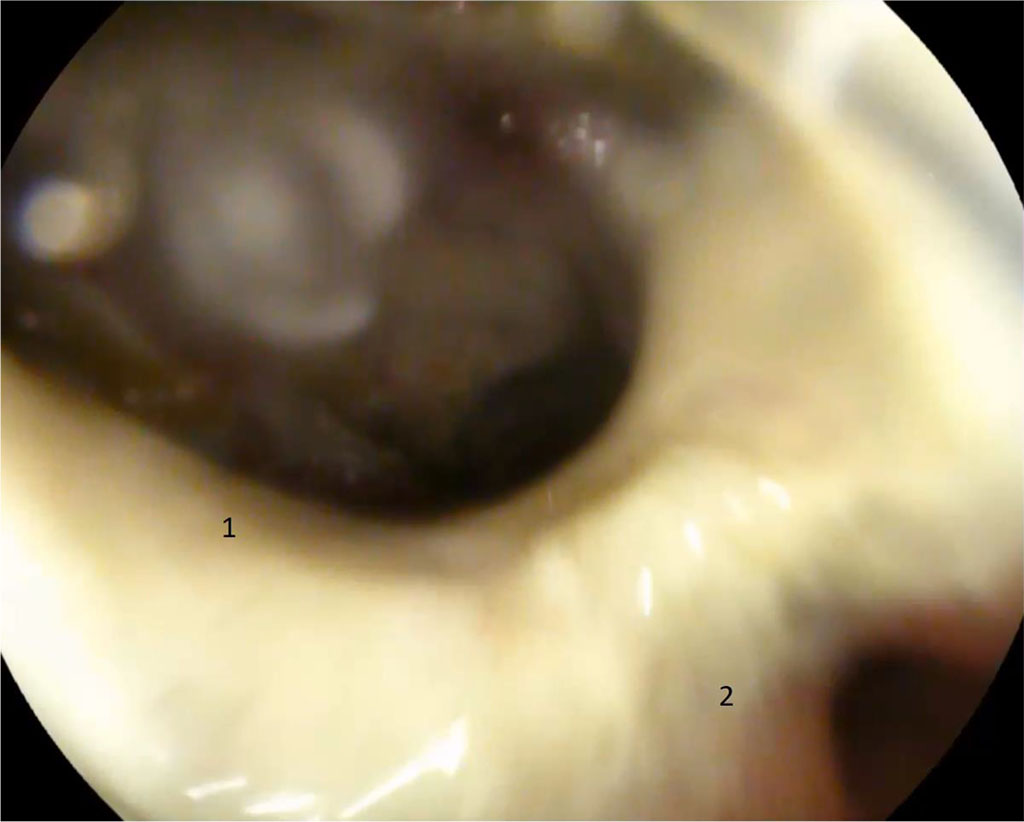
Figure 1. 4 days after surgery. 1 — gastric fistula along the suture line with an orifice into the abdominal abscess; 2 — pyloric part, orifice into the duodenum (explanations in the text)
Since the pyloric part of the stomach was gaping, we refused to administer botulinum toxin, and we also did not perform stenting of the lumen in the area of the defect in the remnant stomach. At the same time, with ultrasound control, a drainage was placed in the abdominal cavity to the area of the defect. The patient was given totally parenteral nutrition and antibiotic therapy with broad-spectrum drugs. After 14 days, the drainage in the abdominal cavity stopped functioning. Fistulography showed that it was not in the abscess cavity; according to endoscopic fistulography, hermetic sealing was achieved.
Day 21, after endoscopic sanitation of the abscess cavity (once every 2 days) on an outpatient basis. Further, a significant decrease in size of the abscess cavity and fistula orifice was noted. Fig. 2.
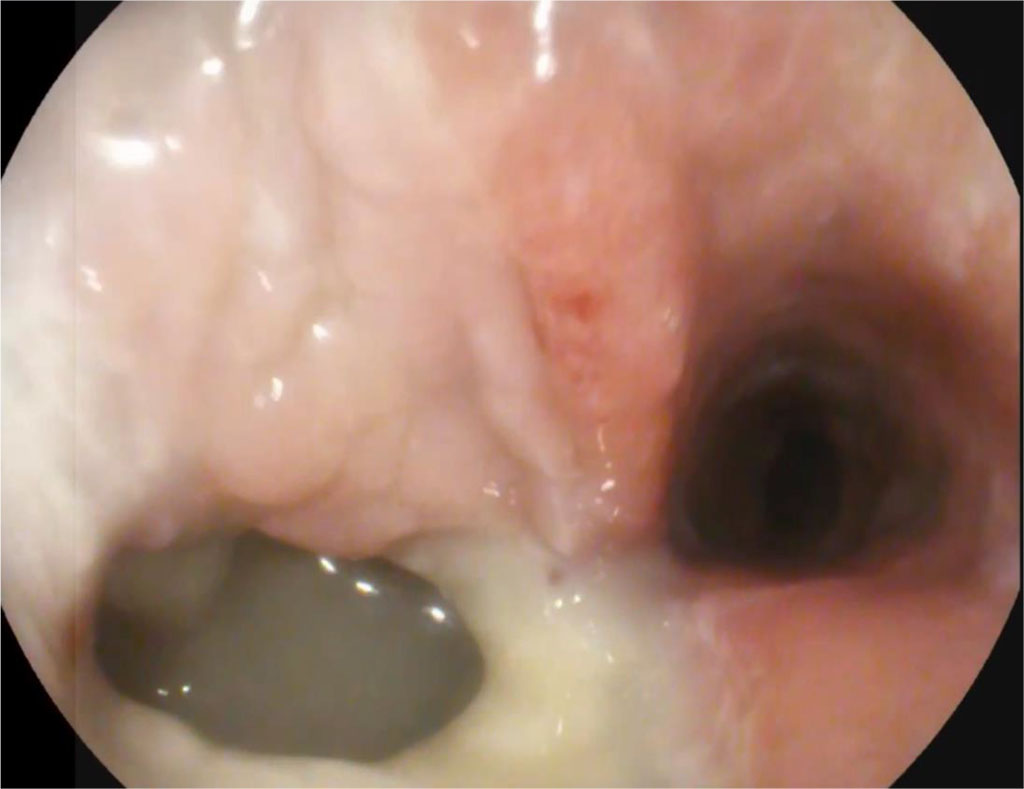
Figure 2. 21 days after surgery (explanations in the text)
After 1.5 months, the fistula was almost completely closed with granulations. Fig. 3.
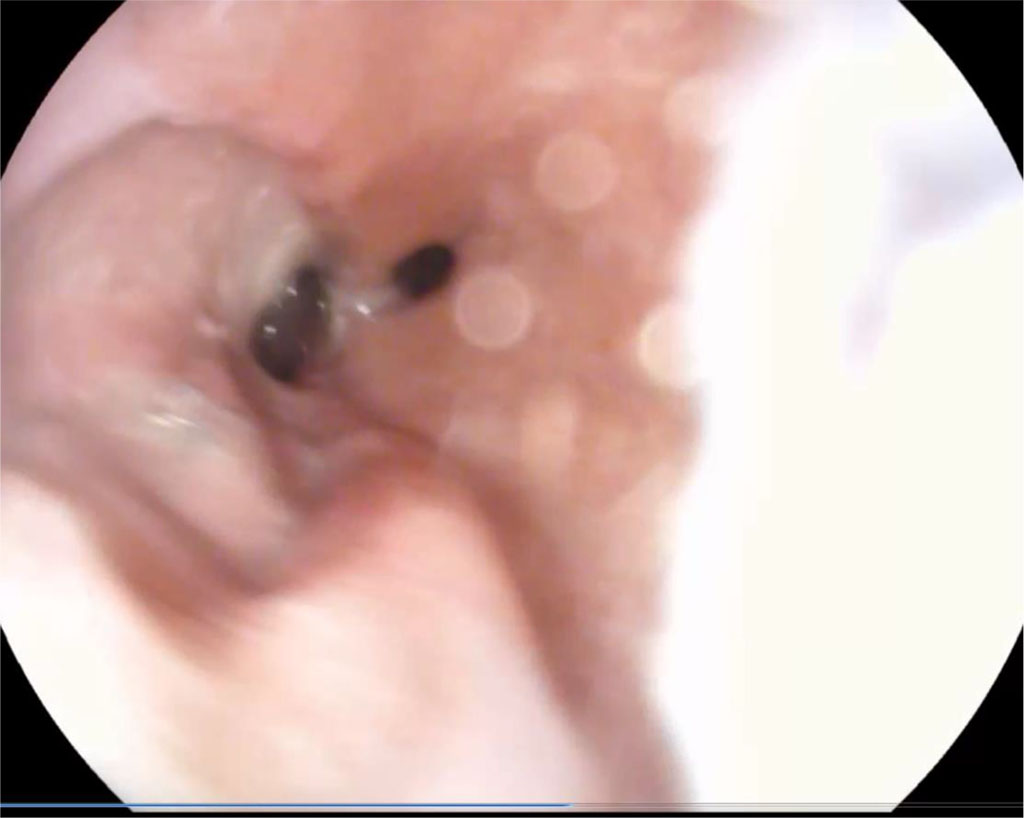
Figure 3. 1.5 months after surgery (explanations in the text)
After 2 months, the fistula was completely closed. The patient was discharged with full recovery. After 6 months, EGD revealed cicatrical changes; body weight loss: 55 kg.
The problem of treatment of the mechanical suture failure of the stomach has not been completely solved. A number of authors suggest stenting the lumen of the gastrointestinal tract, others suggest using botulinum toxin to accelerate the evacuation of food from the gastric stump, and still others suggest using active aspiration through a nasogastric tube and the abscess cavity. In our case, we demonstrated the possibility of combined minimally invasive treatment of this surgical pathology.