- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
DOI 10.35630/2022/12/psy.ro.12
Background: Pneumonia remains an important cause of morbidity and mortality at pediatric age (estimated by UNICEF at 3 million child deaths per year worldwide). Although the etiology of pneumonia is well known, in many patients the exact pathogen is not identified after routine diagnostic workup. We present three cases with complications. that were diagnosed and treated in our Pneumology Clinic. The aim of this case series is to point out the challenges in diagnosing and managing such diseases in children. The evolution of the three cases was difficult, all children needed ICU care. Cultures collected for all of them were negative, probably secondary to the fact that they received antibiotic treatment at home, before carrying out tests. Yet, after receiving broad-spectrum antibiotics and supportive treatment the evolution was favorable in the end. Conclusion: Despite progresses that were made in the last century concerning the antibiotic treatment in pneumonia there are still cases that develop severe complications that require multidisciplinary approach.
Keywords: child, complicated pneumonia, antibiotics.
Pneumonia remains the leading cause of death in children under five worldwide. It accounts for about 1.6 million deaths a year in this age group - 18% of all deaths among children under five (Darby et al., 2017; Nolan et al., 2018).
Even in present times pneumonia remains a public health problem because of its great morbidity and mortality as well as high economic burden worldwide (Bhutta et al., 2013; Falup-Pecurariu et al, 2017; Oestergaard et al., 2011; Pabary & Balfour-Lynn, 2013; UNICEF, 2018).
Many children are treated as outpatients and the proportion of children presenting to the emergency department with pneumonia who are then hospitalized ranges from 19 to 69%. Therefore, pneumonia remains an important cause of hospitalization among unvaccinated children in developed countries (Lupu et al., 2017; Osowicki & Steer, 2019).
In the last two decades it has been described a growth trend of complicated pneumonia, despite of declining rates of community acquired pneumonia in children (Darby et al., 2017).
The focus of this article is on complicated pneumonia which develops not only in children with underlying diagnosis and a low immune status, but also in a small proportion of immunocompetent children.
A child with community acquired pneumonia can develop both local and systemic complications. Usually, local complications refer to pleural effusion/empyema, pericardial effusion, lung abscess, necrotizing pneumonia and atelectasis (Arbo et al., 2019; Osowicki & Steer, 2019; Pabary & Balfour-Lynn, 2013; van Werkhoven & Huijts, 2018).
The most common systemic complications are severe sepsis, acute respiratory distress syndrome, syndrome of inappropriate antidiuretic hormone secretion, hemolytic uremic syndrome, disseminated intravascular coagulation and secondary thrombocytosis. (Arbo et al., 2019; Nolan et al., 2018; van Werkhoven & Huijts, 2018)
We present three cases of pneumonia with unusual evolution, that was not predicted by the minimal symptoms from the onset.
Case 1: 1year and 9months female, presented for fever, dyspnea, productive cough, lack of appetite. At admission she presented influenced status, extreme irritability to the examination, pale skin, expiratory dyspnea, expiratory grunting, increased respiratory rate (41 respiration per minute), intercostal retraction, SaO2 (-) = 93%.
Blood tests showed: increased leukocyte number with predominant neutrophils, anemia, low iron level, positive inflammatory syndrome, negative nasal and pharyngeal exudate.
Chest X-ray showed medium intensity round opacity, bilateral perihilar interstitial infiltration, more significant on the left lung, clear costo-diafragmatic sinuses.
Treatment and evolution: We started antiobio-therapy with Ceftriaxone and Gentamicin and symptomatic treatment with Hydrocortisone hemisuccinate, Acetylcysteine, nebulization and intravenous hydration with favorable evolution (remission of fever, rare cough, but persistence of dyspnea).
In the third day of treatment, we noticed important abdominal distention, worsening dyspnea, lack of stool of about 48 hours. Surgical consultation recommended simple abdominal radiography. Abdominal X-ray description was suggestive for sub occlusive syndrome. (Fig 1)
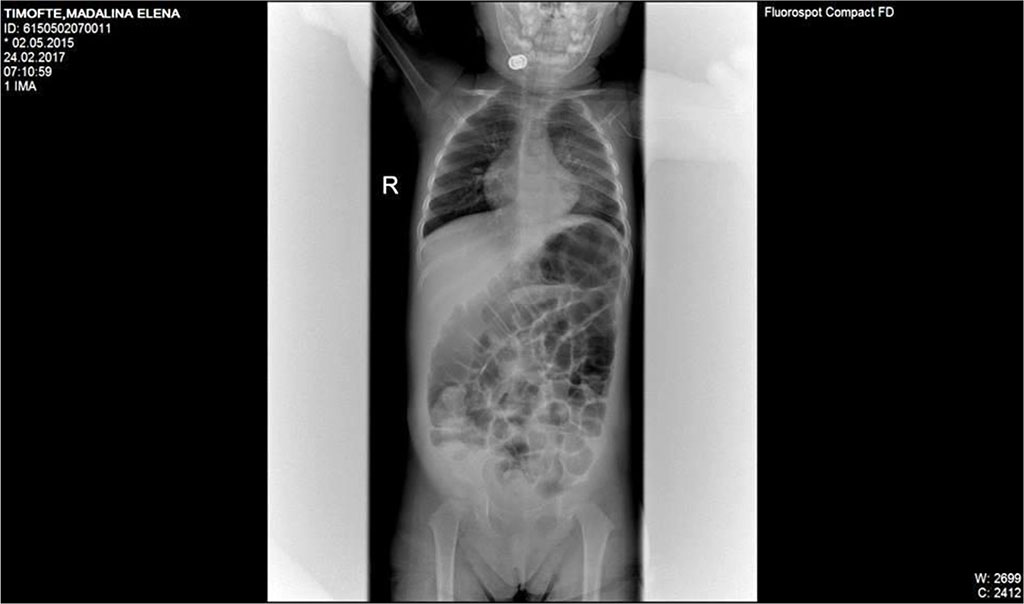
Fig.1. Dilated bowel loops, two c on the left iliac fossa; absence of pneumoperitoneum
Therefore, we have continued treatment only with ceftriaxone and intravenous rehydration, so that the sub occlusive syndrome was resolved, respiratory symptomatology also resolved, but the child maintained discrete dyspnea. Although, the child maintained influenced status, pale skin and irritability.
In evolution, by repeating blood tests we found a minimal increase in leukocyte number and neutrophils and erythrocyte sedimentation rate and C-reactive protein with high values.
Thus, we’ve decided to change from Ceftriaxone to Piperacillin/ Tazobactam. With this new treatment the child had a good evolution both clinical and biological (inflammatory markers decreased significantly). After 8 days with this new antibiotic treatment, the patient resumes symptoms of productive cough, dyspnea, wheeze and rhonchi across the left lung area. Repeated blood tests showed an important increase of CRP. Repeating the X-ray, we saw a transparent lesion of 35/24mm, circled by a 3mm peripheric opaque ring located in the posterior side of left-superior lobe. (Fig.2)
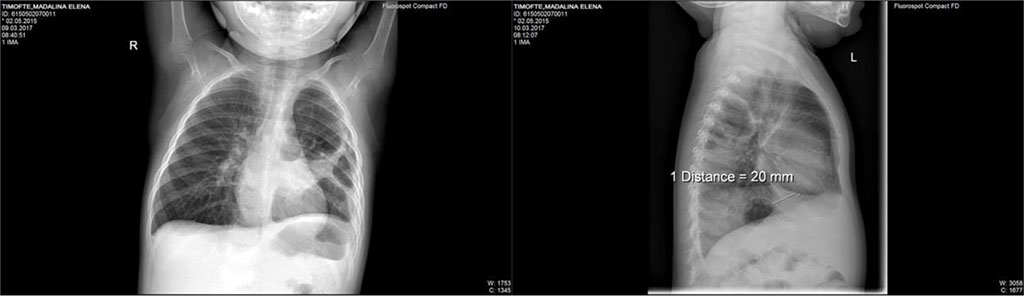
Fig. 2. Transparent lesion of 35/24mm, circled by a 3mm peripheric opaque ring located in the posterior side of left-superior lobe; two air-fluid levels of 8mm and respectively, 40mm located at the posterior basis of left lung
Considering the clinical and paraclinical evolution we have raised the assumption of staphylococcal pneumonia. So, we’ve started treatment with Teicoplanin, Meropenem and Metronidazole with good evolution.
Control chest X-ray described mild interstital infiltrate of both lungs; two radiotransparent lesions that appear to communicate onthe superior located near heart.
The patient had a slow favorable evolution, being hospitalized for about 6 weeks.
Case 2: Female child, 8 years old, presented for fever and chest pain. The disease started 5 days before presentation with fever, vomiting and semi-solid stools.
At admission the patient presented relatively good general status, afebrile, but she had dyspnea, diminished vesicular breath sounds at the base of right lung and dull percussion on the same side.
Blood test showed an increased number of leukocytes (37170/mmc) with predominance of neutrophils (92%) and an important inflammatory syndrome (CRP=252mg/l). Chest x-ray at the onset showed an opacity located on the upper right pulmonary lobe and presence of fluid in the right costo-diafragmatic sinus (Fig.4).
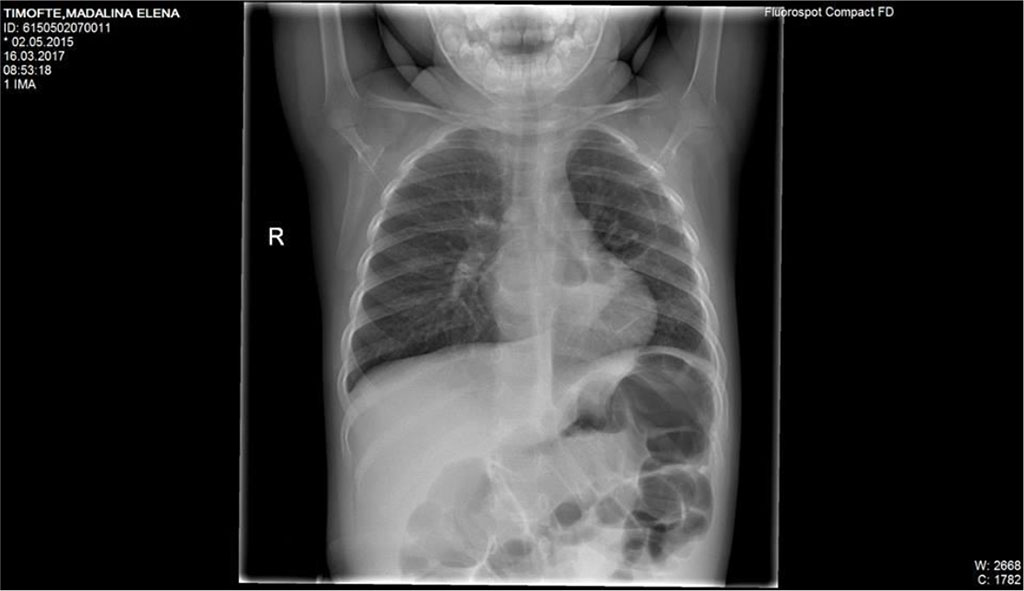
Fig.3 Mild interstital infiltrate of both lungs; pulmonary two radiotransparent lesions that appear near hearth
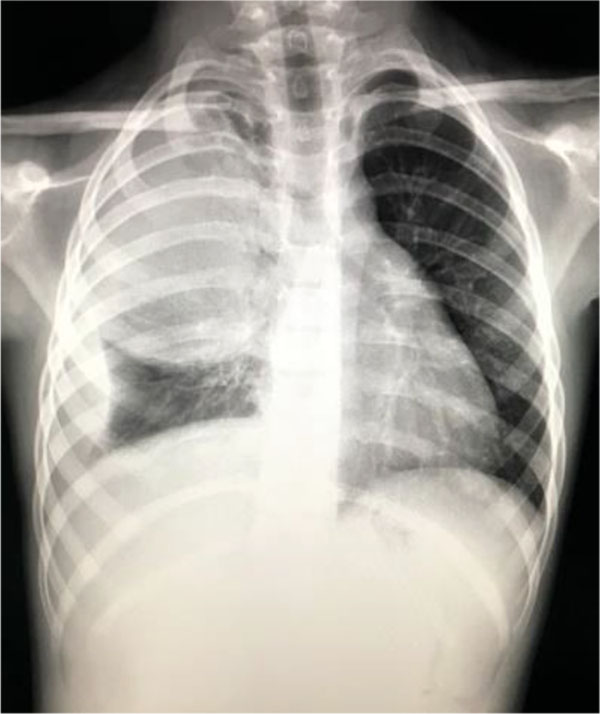
Fig. 4. Opacity located on the upper right pulmonary lobe and presence of fluid in the right costo-diafragmatic sinus
We’ve started the treatment with Ceftriaxone and Gentamicin with unfavorable evolution, increasing chest pain and dyspnea. Our second option was an association between Piperacillin/ Tazobactam without improvement of general state and maintaining of abnormal blood tests. Therefore, thoracic computed tomography was performed (Fig 5,6,7). It revealed the presence of right Pyo-pneumothorax. In that case a pleural drainage was performed in association with large spectrum antibiotic therapy (Meropenem, Linezolid, Metronidazole).
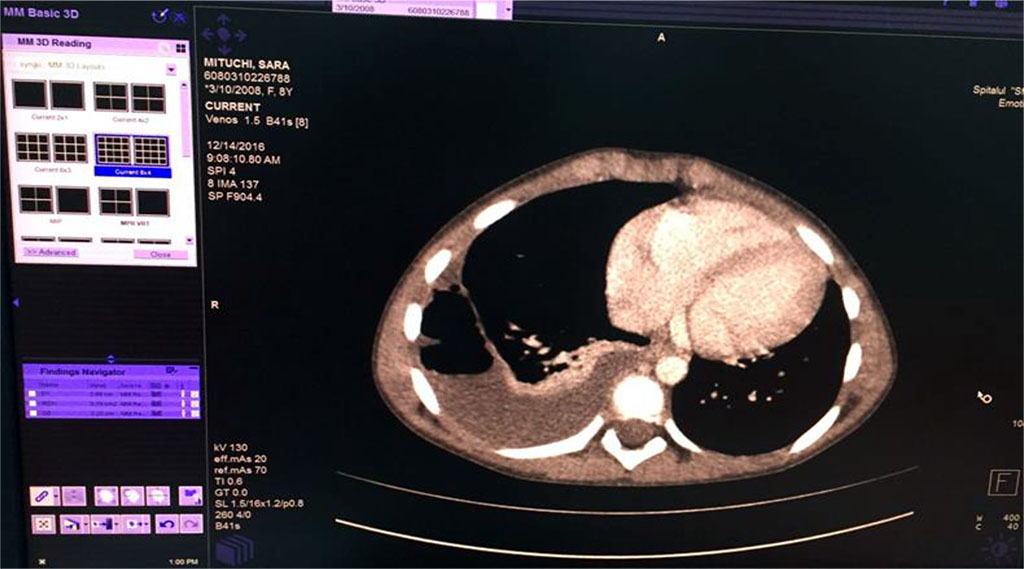
Fig. 5 Right empyema with the thickness of fluid of 27 mm and 22mm thickness of air level
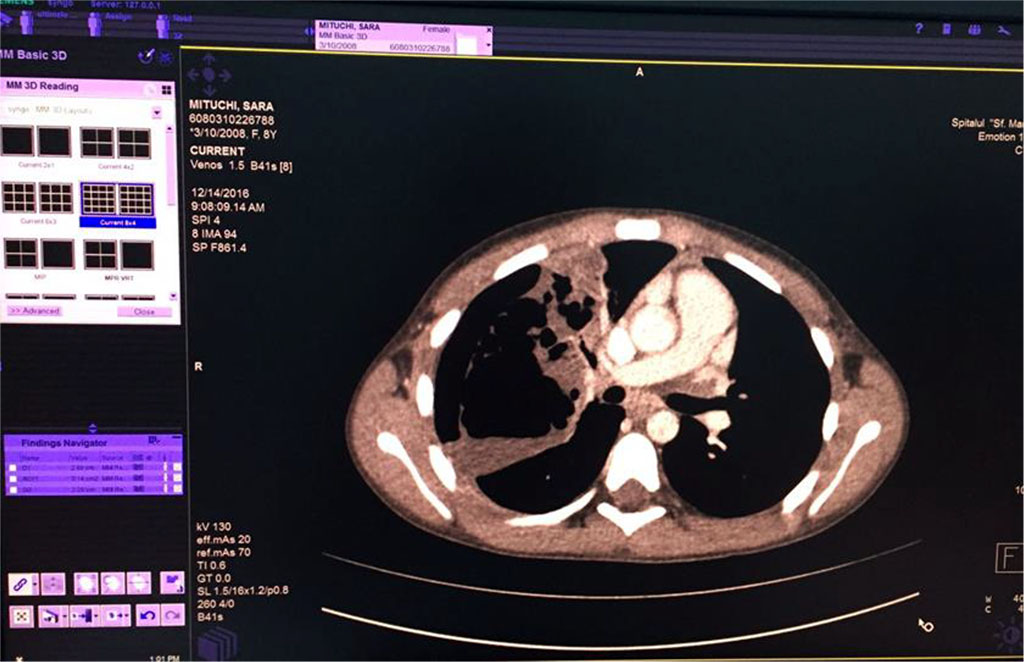
Fig. 6 Air bubbles within the fluid colection of the right lung
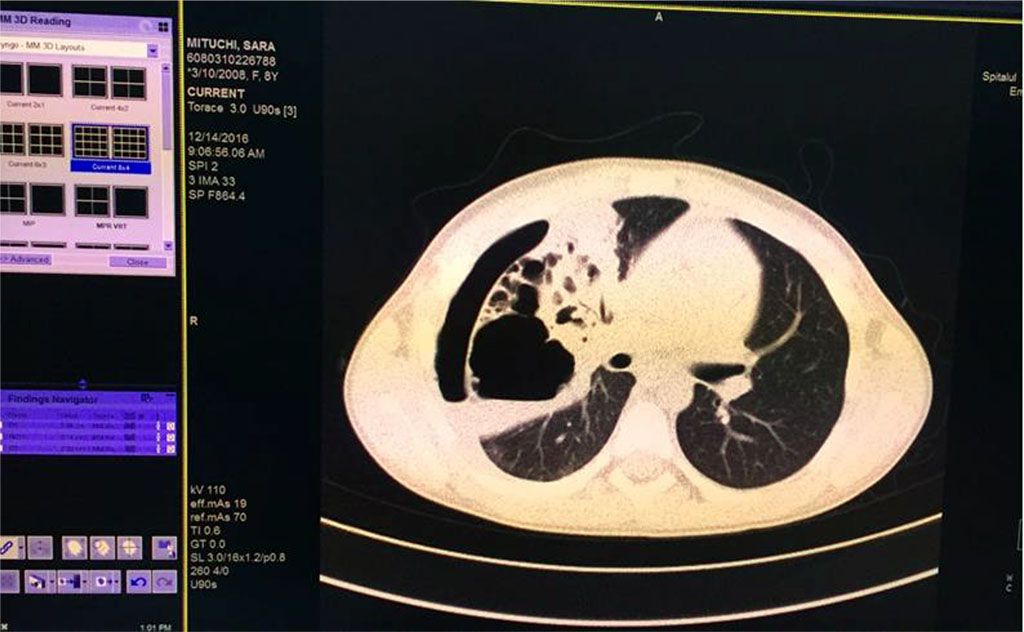
Fig. 7. Pulmonary consolidation, inhomogenous, with multiple aeric cysts with sizes from 11mm to 42.3mm
Seven days after the pleural drainage, another chest x-ray was performed. It revealed the maintaining of a non-homogeneous opacity in medium 2/3 of the right hemithorax (Fig. 8). Thus, a video-assisted thoracoscopy (VAT) pleuropulmonary decortication was done (Fig. 9). A subsequent chest x-ray revealed an ameliorated image (Fig. 10). Post-operatory evolution was slowly favorable despite the association between large spectrum antibiotic therapy and systemic antifungal therapy. The patient also needed supportive with rehydration perfusion, immunotherapy and blood transfusion for about one month.
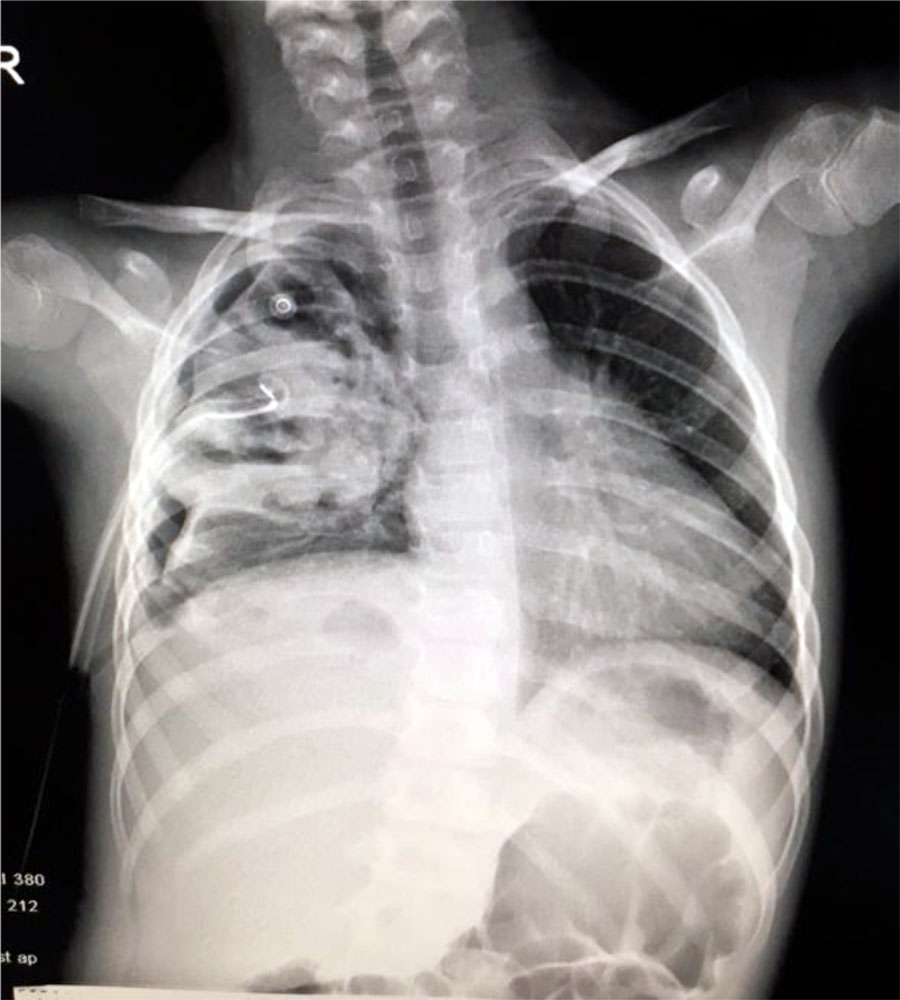
Fig. 8. Non-homogeneous opacity in medium 2/3 of the right hemithorax
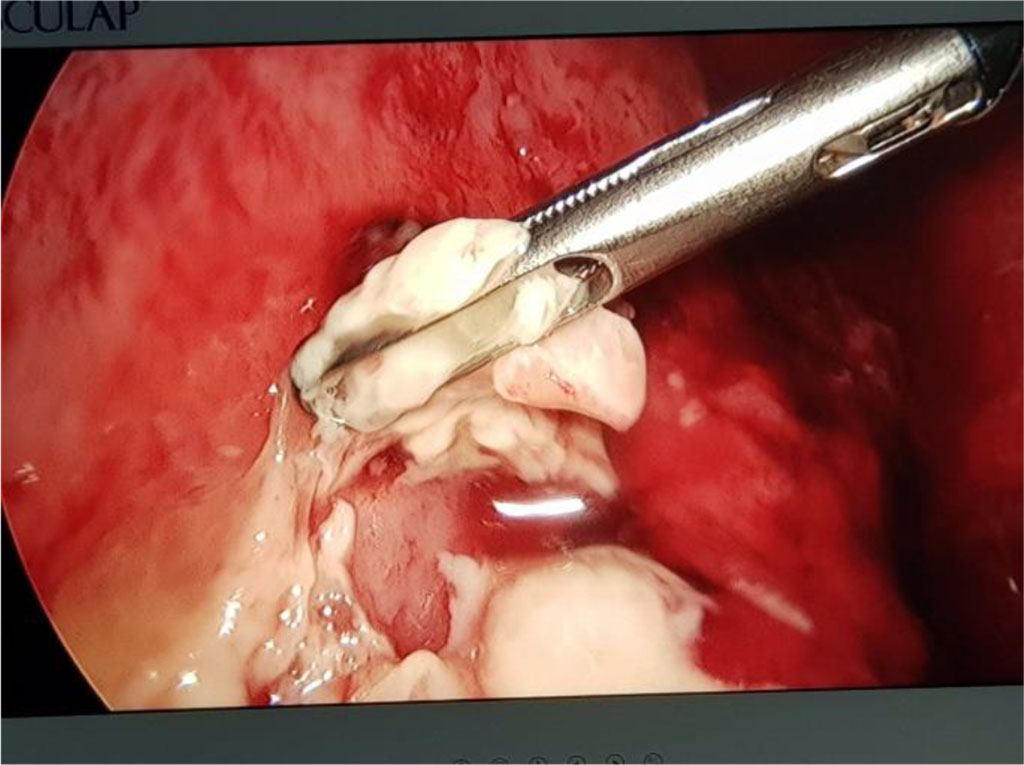
Fig. 9. VAT for pleuropulmonary empyema
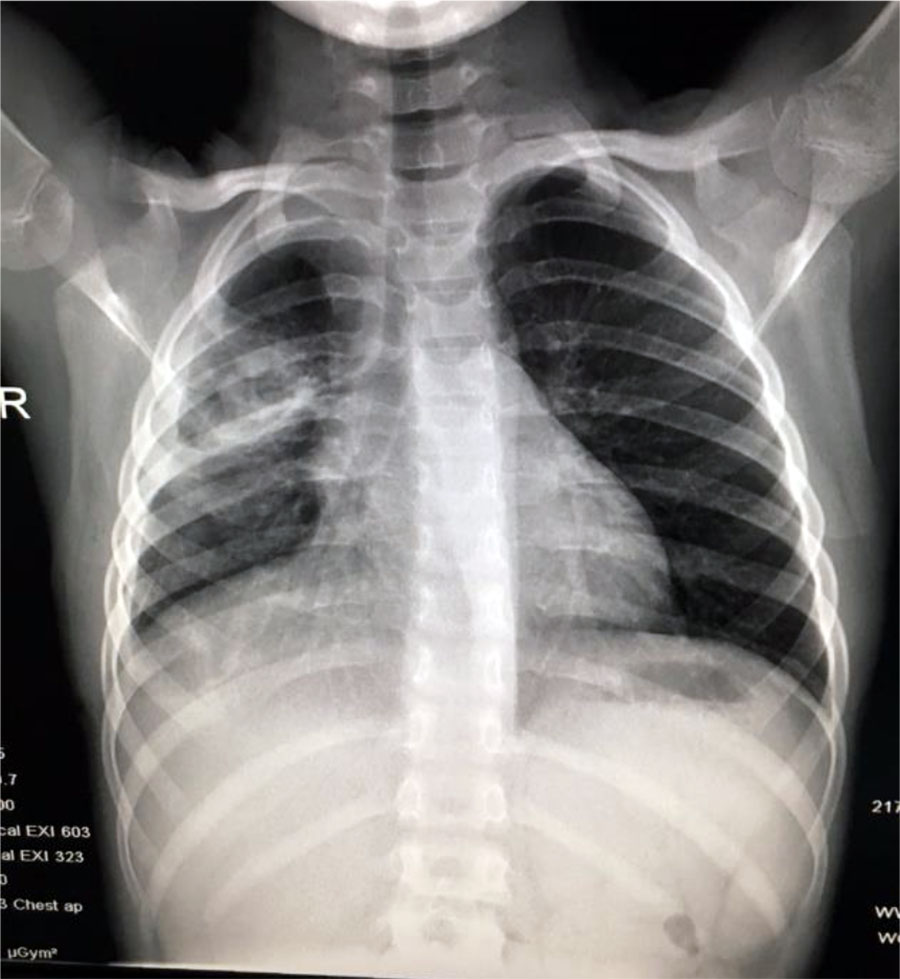
Fig. 10. Same case as in Fig 4-9 after 1 month of treatment
Case 3: A 8-year-old female child presented for fever, sore throat, dysphagia and irritative cough. The symptoms started 3 days before the admission.
The medical history of the patient revealed that she has been diagnosed with acute myeloid leukemia (positive FLT3-ITD mutation – associated with unfavorable prognosis), in remission for 1,5 years. At admission she presented influenced general status, pale skin, clinical aspect of ulcer-necrotic tonsillitis, fever, irritative cough, vesicular breath sounds, without abnormal breath sounds. Blood tests revealed an increased number of leukocytes (34870/mmc) with predominance of neutrophils (80%) and an important inflammatory syndrome (CRP=126mg/l). Bone marrow aspirate revealed late medullary relapse (42% blasts). In that case we’ve started broad spectrum antibiotics (Meropenem, Ciprofloxacin and Metronidazole) that determined the relieving of acute symptoms. After that, chemotherapy was started with low dose cytarabine. In the 11th day of hospitalization, the patient developed high fever, shortness of breath, productive cough and severe post chemotherapy neutropenia (20/mmc). Chest x-ray was consistent for bronchopneumonia. (Fig.11) Therefore, she received treatment with Linezolid and Teicoplanin for the next 10 days. Despite this therapy, the girl had no clinical improvement and maintained respiratory symptoms. For the next 5 days she received Linezolid, Teicoplanin and Fluconazole with poor clinical and radiologic evolution. For the next 7 days we’ve changed Fluconazole with Voriconazole. This treatment having a major impact on both clinical and radiological evolution - regression of codensation and the disapearance of the other 2 opacities.
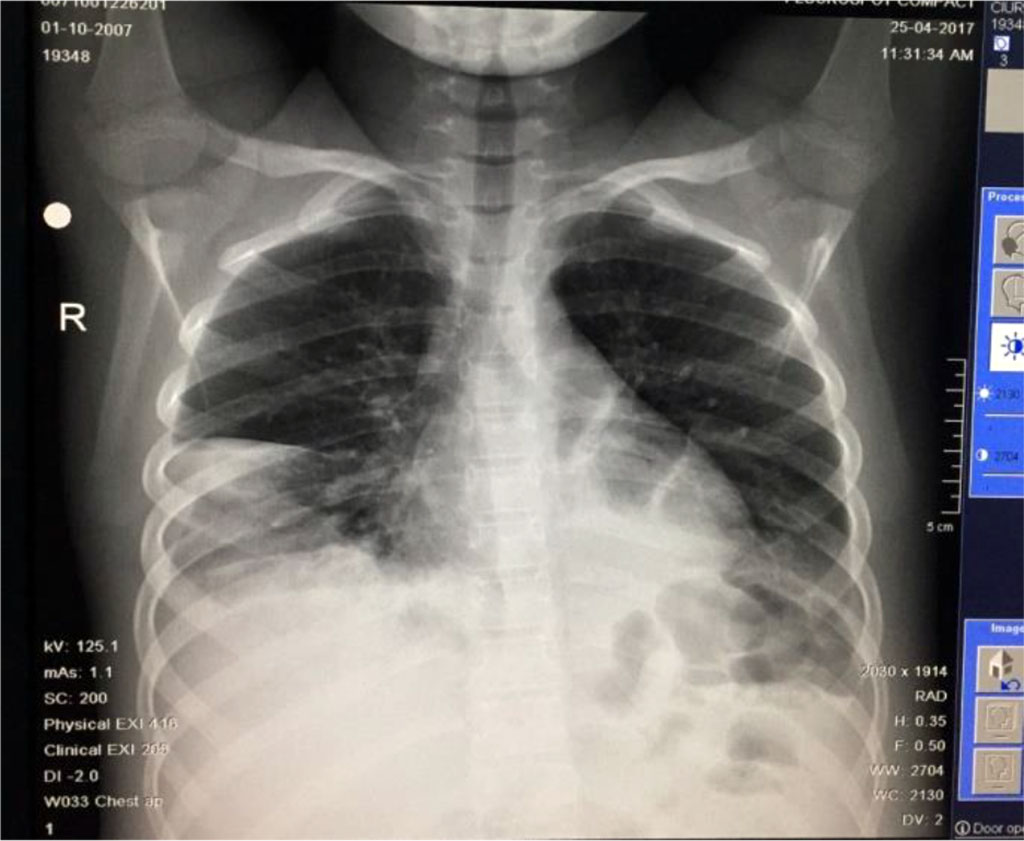
Fig. 11 Medium intensity opacity, inhomogenous, located at the base of right lung and 2 other opacities located retrocardiac
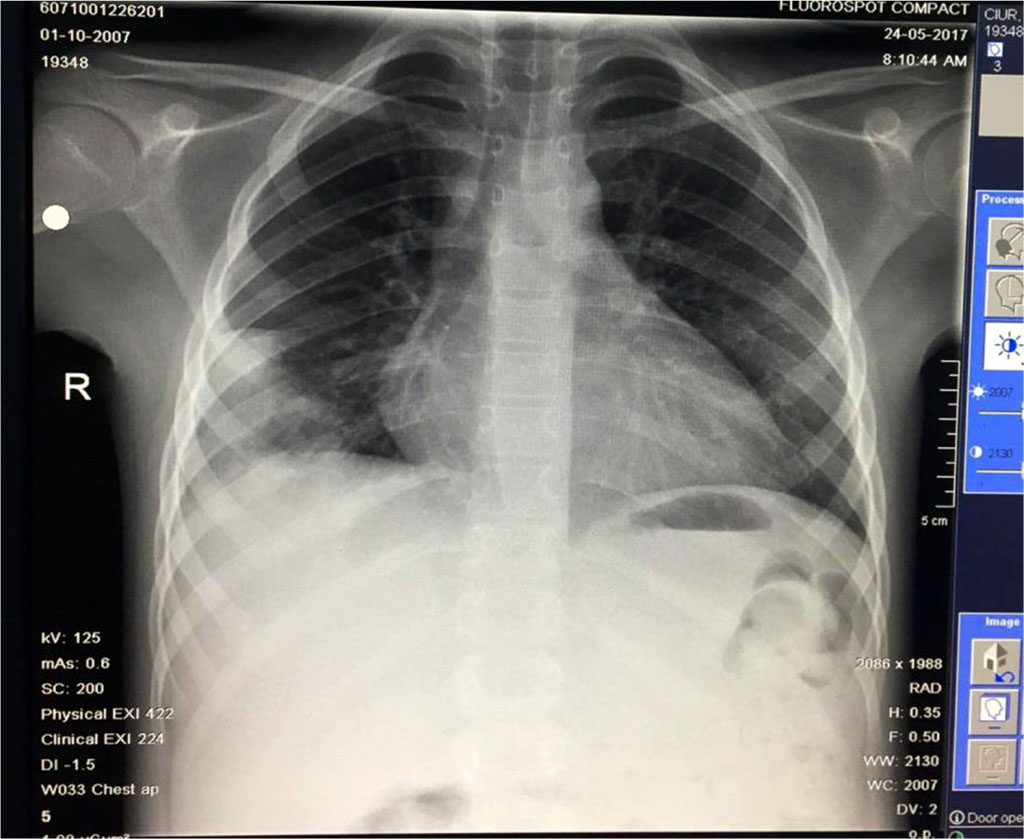
Fig.12 Slow favorable evolution: regression of codensation and the disapearance of the other opacities
Case 1: Apparent immunocompetent child with bronchopneumonia with difficult evolution despite of broad-spectrum antibiotics. Persistent abnormalities from the chest x-rays raised suspicion of preexistent cyst on a possible bronchial malformation but the computed tomography performed at 1 month distance invalidated this assumption.
Case 2: Older child, without medical history, that developed parapneumonic pleural effusion, pulmonary abscess and empyema. In this case, the simple thoracic drainage was not efficient, and the patient needed pleuropulmonary decortication. The minimally invasive procedure (VAT) is recommended and was applied in this case with a good outcome, without the sequelae of a thoracotomy.
Case 3: Immunosuppressed child, known with acute myeloid leukemia (positive FLT3-ITD mutation), with late medullary relapse that developed probably a fungal respiratory pneumonia since she responded to antifungal drugs.
The evolution of the three cases was difficult, all children needing ICU care. Cultures collected for all of them came out negative, probably secondary to the fact that they received antibiotic treatment at home, before carrying out tests. Yet, after receiving broad spectrum antibiotics and supportive treatment the evolution was favorable in the end.
Complicated pneumonia is an important paediatric problem. The choice of the antibiotic is made based on few available data and suggested recommendations from guidelines. Even though the diagnostic tests are advanced, and the immunization coverage is increased as well as treatment options that are available nowadays, the mortality remains high, particularly in developing countries (Arbo et al., 2019; Oestergaard et al., 2011).
Antibiotic therapy is probably the most important aspect of treatment in pneumonia, but supportive care has a role to play in the recovery of the patients diagnosed with this pathology. Yet there are various recommendations of international guidelines for antibiotic therapy in pneumonia. The optimal choice and duration of antibiotic treatment is still debated (Gupta et al., 2018, Haji et al., 2018; Pabary & Balfour-Lynn, 2013)
Empirical antibiotic treatment is still valid in clinical management of pneumonia cases both in children and adults (Arbo et al., 2019; Gupta et al., 2018).
The problem of antibiotic resistance among various pathogens in pneumonia is widely recognized. (Gupta et al., 2018) Over time a big number of drugs have been developed against drug-resistant pathogens and some drugs are still developing. Reasonable use of antibiotics, starting with narrow spectrum drugs and the shortest possible duration may help in reducing the drug resistance (Arbo et al., 2019; Osowicki & Steer, 2019).
Complications such as emphysema and lung abscess often need surgery procedures in addition to antibiotic treatment such as video-assisted thoracoscopy or insertion of percutaneous small-bore drainage with instillation of fibrinolytics (Arbo et al., 2019; Gupta et al., 2018; Haji et al., 2018; Knebel et al., 2018; Shan et al., 2019).
Repeated ultrasounds may be required in the monitoring these patients, in the case of clinical deterioration as fluid may accumulate rapidly (Knebel et al., 2018; Williams et al., 2016). The radiologic image may remain abnormal for up to 6 months after treatment. Usually children fully recover without long term sequelae, but complications are possible such as: fistulae (broncho-pleural or cutaneous), bacteremia, endocarditis, persistant lobar colapse (Knebel et al., 2018; Jiang et al., 2017; Shan et al., 2019; Vendemmia et al., 2019; Williams et al., 2016).
Conclusion: Despite progress that was made in the last century concerning the antibiotic treatment in pneumonia there are still cases that develop severe complications that require multidisciplinary approach.