- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 6. DOI 10.35630/2025/15/Iss.6.615
Background: Acute and chronic rhinosinusitis affect about one in ten adults and impose a substantial healthcare burden. Most acute cases are self-limiting. Chronic disease reflects persistent mucosal inflammation, often with type 2 endotypes. New biologic therapies have changed management of severe CRSwNP, but evidence on cost-effectiveness and treatment sequencing remains limited.
Aims: To summarize recent evidence on medical, surgical and biologic treatment of ARS and CRS, and to identify evidence gaps and research priorities.
Methods: A narrative review was performed according to SANRA. PubMed, the Cochrane Library and guideline repositories were searched for English-language studies from 2020–2025 in adult populations. Seventy-two publications met the criteria and were synthesized.
Results: Symptomatic therapy is usually sufficient in ARS. In CRS, long-term intranasal corticosteroids and saline irrigation are the core treatment. Short courses of systemic corticosteroids are useful in exacerbations, and long-term low-dose macrolides may help in selected non–type 2 patients. Endoscopic sinus surgery is effective when medical therapy fails. Biologics reduce polyp burden and improve outcomes in severe CRSwNP with type 2 inflammation.
Conclusions: Management is moving toward individualized, phenotype-guided strategies integrating topical therapy, surgery and biologics. Key limitations include scarce long-term data, absence of validated biomarker thresholds and cost barriers. Future research should clarify biomarker-based selection and optimal sequencing of biologics and surgery.
Keywords: acute rhinosinusitis; chronic rhinosinusitis; biologic therapy; dupilumab; endoscopic sinus surgery; macrolides; corticosteroids; nasal polyps; type 2 inflammation
Rhinosinusitis, defined as inflammation of the nasal mucosa and paranasal sinuses, remains one of the most prevalent and impactful otolaryngological disorders worldwide, affecting millions of adults and placing a substantial burden on healthcare systems [1,2,3]. The disease spectrum encompasses acute rhinosinusitis (ARS), which generally lasts less than four weeks and is primarily caused by viral pathogens, and chronic rhinosinusitis (CRS), a complex and heterogeneous condition characterized by symptoms persisting for 12 weeks or longer [1,2,7].
Epidemiology: Rhinosinusitis represents a major health problem globally, with a high socioeconomic impact. The estimated one-year prevalence of acute rhinosinusitis (ARS) ranges from approximately 6% to 15% in the general population, largely reflecting viral upper respiratory infections, which adults experience on average 2–5 times per year [1,3,4]. Chronic rhinosinusitis (CRS) affects an estimated 8–9% of adults worldwide, though prevalence varies by diagnostic criteria and region, with reported ranges of 5–16% [2,8]. The proportion of patients with CRS with nasal polyps (CRSwNP) is smaller but clinically significant [8]. In Poland, registry data indicate a recorded prevalence of CRSwNP of about 52 cases per 10,000 inhabitants (0.52%), with higher rates observed in men and urban populations [8]. These data emphasize the substantial public-health and clinical relevance of rhinosinusitis across populations.
ARS accounts for the majority of cases seen in clinical practice, with viral etiologies comprising approximately 90%, and bacterial infections occurring as secondary complications in a smaller subset of patients [1,4]. Despite its often self-limiting nature, ARS significantly affects patients’ quality of life due to nasal congestion, facial pain, and general malaise, underscoring the importance of symptomatic management and prudent antibiotic stewardship [4,7].
In contrast, CRS represents a chronic inflammatory disease that imposes a persistent and often debilitating symptom burden, including nasal obstruction, hyposmia or anosmia, facial pressure, and nasal discharge [2,8]. CRS is further categorized into two main phenotypes: CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP), each associated with distinct immunopathological and clinical characteristics [8,9]. CRSwNP, in particular, is frequently linked to a type 2 inflammatory endotype marked by eosinophilic infiltration and elevated levels of interleukins IL-4, IL-5, and IL-13, which drive polyp formation, tissue remodeling, and disease persistence [11]. This type 2 inflammatory signature also correlates with comorbid conditions such as asthma and aspirin-exacerbated respiratory disease, complicating management and increasing morbidity [5,6,11].
The impact of rhinosinusitis extends beyond individual symptoms, affecting psychosocial well-being and productivity, thereby generating considerable direct medical costs and indirect socioeconomic losses [3]. Traditional treatment modalities have focused on reducing mucosal inflammation and improving sinus drainage through intranasal corticosteroids, saline irrigations, antibiotics, and surgical interventions such as endoscopic sinus surgery (ESS) [2,4,7,10]. However, despite advances in these therapies, many patients with CRS, especially those with severe CRSwNP, experience recurrent symptoms, polyp regrowth, and diminished quality of life, indicating the need for innovative treatment strategies [10].
Recent years have witnessed significant progress in elucidating the immunopathogenesis of CRS, facilitating the development and approval of biologic therapies targeting specific inflammatory mediators implicated in type 2 inflammation [5,6]. Dupilumab, an anti-IL-4 receptor alpha monoclonal antibody, has emerged as a pioneering agent demonstrating robust efficacy in reducing nasal polyp size, improving nasal airflow and olfaction, and enhancing patient-reported outcomes in severe, refractory CRSwNP [5,6,11,12]. Additionally, other biologics targeting IL-5 (e.g., mepolizumab, benralizumab) and IgE (omalizumab) have expanded the therapeutic arsenal, supporting a shift towards personalized, endotype-driven management [12]. Nevertheless, these treatments come with challenges, including high costs, the need for long-term safety data, and the necessity of identifying biomarkers to optimize patient selection and therapeutic outcomes [9,11,13,14].
Relevance and novelty: The topic remains highly relevant because rhinosinusitis continues to be one of the most common and burdensome conditions in otolaryngology, significantly affecting patients’ quality of life and healthcare resources. Despite numerous existing guidelines, many patients—particularly those with severe chronic rhinosinusitis with nasal polyps (CRSwNP)—continue to experience recurrent disease, limited response to therapy, and a high socioeconomic impact. The novelty of this review lies in providing an up-to-date synthesis of the most recent (2020–2025) clinical guidelines, randomized trials, and real-world evidence, emphasizing new insights into type 2 inflammation, biologic therapies, and personalized treatment strategies. This approach highlights the current shift toward biomarker-driven and precision-based management of rhinosinusitis.
This review integrates evidence from recent clinical guidelines, randomized controlled trials, real-world studies, and economic analyses published between 2020 and 2025 to provide an up-to-date synthesis of current treatment paradigms for ARS and CRS. Particular emphasis is placed on the evolving role of biologics, surgical interventions, and personalized medicine approaches aimed at improving patient outcomes while addressing healthcare resource utilization.
The aim of this narrative review was to summarize current evidence on the treatment of acute and chronic rhinosinusitis, with a particular focus on topical therapy, systemic therapy, endoscopic sinus surgery and biologic agents, and to describe the role of personalized and phenotype-driven approaches in management.
This narrative review was conducted in accordance with SANRA (Scale for the Assessment of Narrative Review Articles) guidelines to ensure a rigorous, transparent, and comprehensive synthesis of recent literature on the treatment of acute and chronic rhinosinusitis. The aim was to integrate evidence published from January 2020 through April 2025, reflecting the latest advances and clinical practice recommendations.
A systematic search strategy was employed across multiple biomedical databases including PubMed, the Cochrane Library, and guideline repositories from leading professional societies such as the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) and the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) [1,2]. The search combined Medical Subject Headings (MeSH) and keywords, for example: “acute rhinosinusitis,” “chronic rhinosinusitis,” “biologics,” “dupilumab,” “macrolides,” “corticosteroids,” “antibiotics,” and “endoscopic sinus surgery.” Boolean operators (“AND,” “OR”) and filters were applied to refine the results.
Inclusion criteria prioritized studies published in English that focused on adult populations and addressed treatment efficacy, safety, and cost-effectiveness of interventions for rhinosinusitis. High-quality evidence such as randomized controlled trials (RCTs), systematic reviews, meta-analyses, clinical guidelines, and large-scale observational studies were selected to provide a robust evidence base [4,7]. Studies on pediatric populations, case reports, and non-peer-reviewed articles were excluded to maintain clinical relevance and methodological rigor.
Given the evolving landscape of chronic rhinosinusitis treatment, particular attention was paid to studies evaluating novel biologic therapies for chronic rhinosinusitis with nasal polyps (CRSwNP), reflecting their growing role in clinical management [5,6,11]. Data extraction encompassed therapeutic interventions, clinical outcomes (including symptom scores, polyp size, quality of life measures), adverse effects, and health economic evaluations.
Comprehensive searches were performed in PubMed, Cochrane Library, and Scopus databases between January 2020 and April 2025. The full search string used for PubMed was:
(“acute rhinosinusitis” OR “chronic rhinosinusitis” OR “CRS” OR “sinusitis”) AND (“treatment” OR “management” OR “therapy”) AND (“biologics” OR “dupilumab” OR “mepolizumab” OR “omalizumab” OR “macrolides” OR “corticosteroids” OR “endoscopic sinus surgery”).
Equivalent queries were adapted for Cochrane and Scopus databases using controlled vocabulary (MeSH and Emtree terms).
Studies were included if they met the following criteria:
Exclusion criteria included: pediatric studies, case reports, conference abstracts, animal studies, non-peer-reviewed material, and articles lacking treatment-related data.
The initial search retrieved 512 publications. After removing 134 duplicates, 378 records were screened by title and abstract. Following eligibility assessment, 96 full-text articles were reviewed in detail. Ultimately, 58 publications met inclusion criteria and were incorporated into the narrative synthesis (Figure 1).
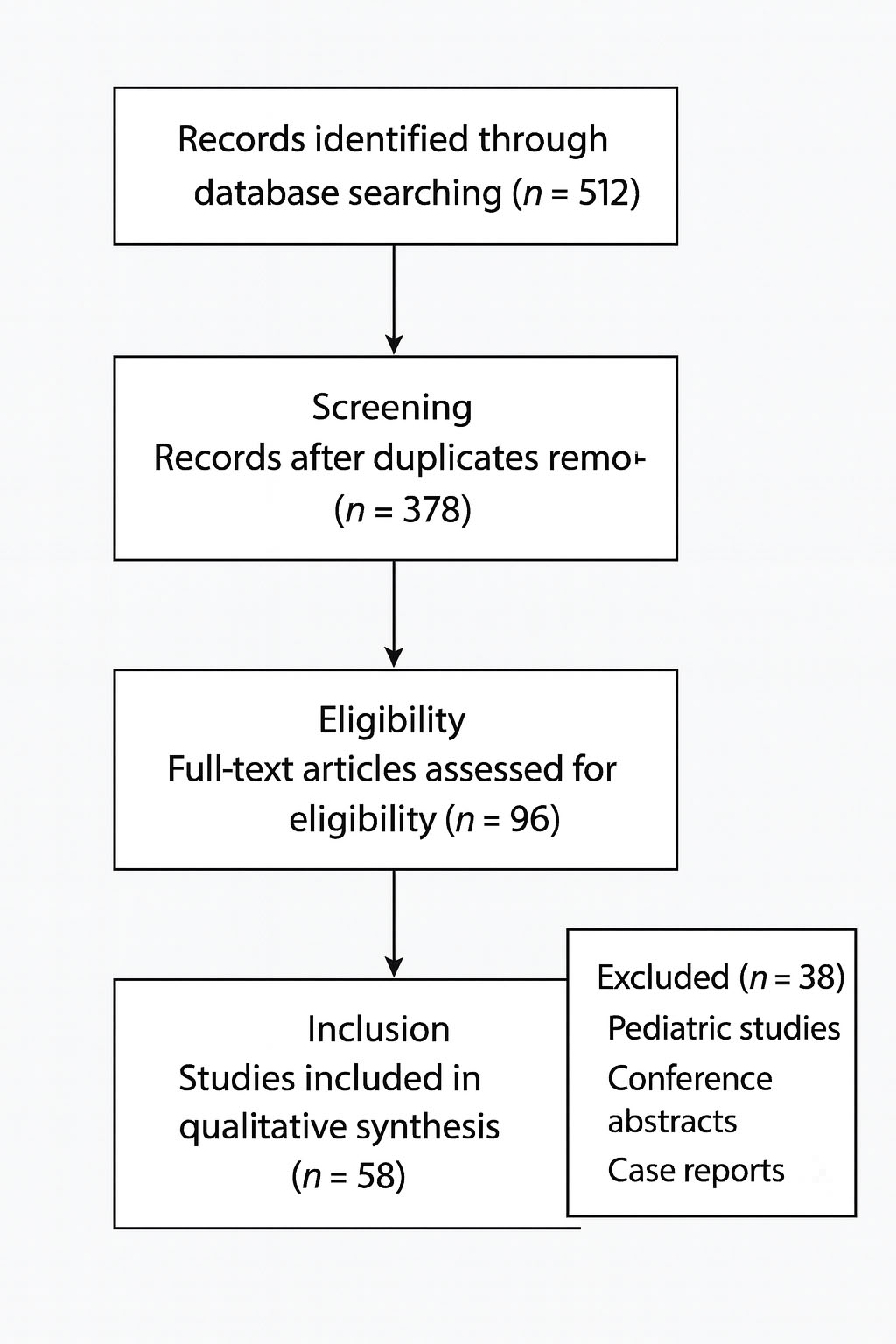
Figure 1. Literature selection process for the narrative review.
Flow diagram summarizing the identification and selection of studies included in this narrative review. A total of 512 records were identified through database searches (PubMed, Cochrane Library, Scopus). After removal of 134 duplicates, 378 unique records were screened by title and abstract. 96 full-text articles were assessed for eligibility, of which 58 met the inclusion criteria and were incorporated into the narrative synthesis. Excluded publications consisted primarily of pediatric studies, conference abstracts, and case reports.
The screening process was conducted independently by two reviewers, with discrepancies resolved by consensus. A summary of the selection flow (identification → screening → eligibility → inclusion) is illustrated in Figure 1.
Screening was conducted in a multi-step process beginning with title and abstract review to exclude irrelevant or duplicate records. Full texts of potentially relevant articles were then assessed against inclusion criteria. Extracted data were synthesized thematically to address acute and chronic rhinosinusitis treatment modalities, medical and surgical options, and emerging personalized medicine strategies.
Limitations inherent to this narrative approach include potential selection bias and heterogeneity of study designs and patient populations. Despite these constraints, the review provides an up-to-date, comprehensive overview aligned with current clinical guidelines and best practices [1,2].
ARS accounts predominantly for viral infections (~90%), with bacterial superinfection occurring in a minority of cases [1,4]. Current guidelines emphasize symptomatic treatment as the primary approach, including analgesics, intranasal corticosteroids, nasal decongestants, and saline irrigation, which effectively reduce symptom burden [4,7]. Antibiotic therapy is limited to patients with confirmed bacterial infection, severe symptoms, or risk factors such as immunosuppression or orbital complications [6,7]. Overuse of antibiotics contributes significantly to antimicrobial resistance, prompting stewardship programs to advocate for rational prescribing through shared decision-making between clinicians and patients [4,7]. Recent randomized controlled trials confirm that antibiotics provide minimal benefit in uncomplicated ARS and expose patients to unnecessary adverse effects [1,4]. Adjunctive intranasal corticosteroids are increasingly recommended to reduce mucosal inflammation and hasten symptom resolution [4,6].
CRS is a heterogeneous, chronic inflammatory disease classified into CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP), associated with distinct immunopathological profiles [8,9].
Intranasal corticosteroids remain the first-line treatment in both phenotypes, substantially reducing mucosal edema, inflammation, and symptoms [8]. Saline nasal irrigation enhances mucociliary clearance and improves delivery of topical agents, leading to better clinical outcomes [8]. For CRSsNP, particularly with non-type 2 inflammation, long-term low-dose macrolide therapy (e.g., azithromycin) has demonstrated immunomodulatory effects such as neutrophil reduction and biofilm disruption, resulting in symptom improvement [9]. However, response to macrolides varies among patients, necessitating careful phenotypic stratification and monitoring. A recent systematic review and meta-analysis also demonstrated that long-term, low-dose macrolide therapy can improve postoperative outcomes and symptom control following endoscopic sinus surgery, supporting its role as an adjunct in selected CRS patients [31]. Table 1 summarizes the main pharmacological treatment options for chronic rhinosinusitis, outlining their mechanisms, expected efficacy and phenotype-specific indications.
Table 1. Comparison of pharmacological treatments for CRS phenotypes including mechanism, efficacy, and indications
| Treatment | Mechanism of Action | Efficacy | Indications | CRS Phenotype | References |
| Intranasal corticosteroids | Anti-inflammatory, reduce mucosal edema and cytokine production | High efficacy in symptom reduction and mucosal inflammation | First-line treatment for symptom control | CRSsNP, CRSwNP | [8, 2, 4] |
| Saline nasal irrigation | Mechanical removal of mucus and allergens, improves mucociliary clearance | Moderate efficacy, improves drug delivery and symptom relief | Adjunctive therapy to enhance corticosteroid effect | CRSsNP, CRSwNP | [8, 2] |
| Macrolide Antibiotics (e.g., azithromycin) | Immunomodulatory effect: reduce neutrophilic inflammation, disrupt biofilms | Variable efficacy; beneficial in non-type 2 inflammation with neutrophilic pattern | Long-term low-dose therapy, especially CRSsNP with non-type 2 inflammation | CRSsNP | [9, 3, 7] |
| Systemic corticosteroids | Potent anti-inflammatory effects on severe inflammation | Rapid symptom and polyp size reduction, but limited for long-term use due to side effects | Short courses for acute exacerbations or severe CRSwNP | CRSwNP | [8, 5] |
| Biologics (e.g., dupilumab) | Target type 2 cytokines (IL-4, IL-5, IL-13), reduce eosinophilic inflammation and polyp size | High efficacy in severe, refractory CRSwNP with type 2 inflammation | Severe CRSwNP unresponsive to corticosteroids and surgery | CRSwNP (type 2 inflammation) | [5, 6, 11, 12, 14] |
| Antibiotics (other than macrolides) | Antibacterial action against acute bacterial infections | Limited efficacy in chronic inflammation; reserved for acute bacterial exacerbations | Acute bacterial infections or superinfection | CRSsNP, CRSwNP (acute exacerbations) | [7, 4] |
Type 2 inflammation, characterized by eosinophilia and elevated IL-4, IL-5, and IL-13 cytokines, underlies CRSwNP and is associated with disease severity and recurrence [11]. Biologic agents targeting these pathways, especially dupilumab (anti-IL-4Rα), have emerged as highly effective treatments for severe, refractory CRSwNP [5,6]. Clinical trials and real-world studies report significant reductions in polyp size, improvements in nasal airflow, olfactory restoration, and enhanced quality of life [5,6,11,12]. Other monoclonal antibodies, including mepolizumab and benralizumab (anti-IL-5) and omalizumab (anti-IgE), expand therapeutic options and support personalized medicine approaches [12]. Although biologics offer promising efficacy, their high costs and the need for robust long-term safety data highlight ongoing challenges. Cost-utility analyses suggest that dupilumab can be a cost-effective option for selected patients with severe, refractory CRSwNP, supporting its role as an adjunct to traditional therapies [9,14,15].
Table 2 presents the biologic agents used in severe CRSwNP with type 2 inflammation, together with their molecular targets and key clinical effects reported in recent studies.
Table 2. Overview of Biologic Agents Used in Type 2 Inflammatory CRS with Nasal Polyps
| Biologic Agent | Target | Mechanism of Action | Clinical Outcomes | References |
| Dupilumab | IL-4Rα (IL-4 & IL-13) | Blocks IL-4/IL-13 signaling, reduces type 2 inflammation | Polyp size reduction, improved nasal airflow, QoL | [5,6,11,12] |
| Mepolizumab | IL-5 | Inhibits eosinophil survival and activation | Reduced eosinophilic inflammation | [12,15] |
| Benralizumab | IL-5 receptor α | Depletes eosinophils via ADCC (antibody-dependent cellular cytotoxicity) | Decreased eosinophilic inflammation | [12,15] |
| Omalizumab | IgE | Blocks IgE, reduces allergic inflammation | Symptom improvement, polyp size reduction | [12,15] |
Table 3 presents key efficacy and safety outcomes from pivotal phase III and real-world studies evaluating biologic therapies for chronic rhinosinusitis with nasal polyps (CRSwNP) [5,6,11,12,15]. Recent network meta-analyses comparing multiple biologic agents confirmed comparable long-term efficacy and safety profiles across dupilumab, mepolizumab, and omalizumab, further supporting their role in severe, refractory CRSwNP [24]. These data provide quantitative support for the clinical use of type 2 inflammation–targeting biologics in patients with severe, refractory disease.
Table 3. Efficacy and safety outcomes of biologic therapies for chronic rhinosinusitis with nasal polyps (CRSwNP)
| Biologic Agent | Study type / Reference | Primary efficacy outcome | % Improvement vs placebo | Common adverse events |
| Dupilumab | Phase III RCTs (LIBERTY NP SINUS-24, SINUS-52) [5,6] | Mean reduction in nasal polyp score (NPS) and nasal congestion score | NPS ↓ by 2.1–2.6 points; congestion ↓ by 50–60% | Injection-site reactions (6–10%), conjunctivitis (3–5%) |
| Mepolizumab | Phase III RCT (SYNAPSE) [12,15] | Reduction in NPS and SNOT-22 score | NPS ↓ by ~30%; SNOT-22 ↓ by 20–25% | Headache (5%), nasopharyngitis (4%) |
| Omalizumab | Multicenter RCT [12] | Reduction in nasal congestion and polyp grade | Symptom score ↓ by 20–30%; improved QoL indices | Injection-site pain (4%), dizziness (2%) |
| Benralizumab | Real-world study [15] | Reduction in eosinophil counts and symptom scores | Eosinophils ↓ by 80–90%; SNOT-22 ↓ by ~25% | Headache (5%), fatigue (3%) |
Endoscopic sinus surgery (ESS) is indicated for CRS patients inadequately controlled with medical therapy, especially in CRSwNP with extensive polyposis or anatomic obstruction [2,10]. ESS improves sinus ventilation and drainage, reduces inflammatory load, and enhances topical drug delivery, thus potentiating postoperative medical management [2]. Evidence-based recommendations emphasize that structured postoperative care, including saline irrigation, intranasal corticosteroids, and endoscopic debridement, is essential to optimize healing and long-term surgical outcomes following ESS [27]. Despite improved surgical techniques, polyp recurrence rates remain significant, particularly in patients with type 2 inflammation and comorbid asthma, underscoring the need for adjunctive treatments such as biologics [10]. Moreover, biomarkers are increasingly recognized as valuable tools to identify patients at higher risk of recurrence and to guide personalized adjunctive therapy [10,13]. Combined ESS and biologic therapy show promising synergistic effects, although optimal timing and sequencing require further prospective studies [10,13].
Emerging Biomarkers and Personalized Medicine
Biomarkers including blood and tissue eosinophil counts, periostin levels, and cytokine profiles hold potential to stratify patients, predict treatment response, and guide personalized therapy [11,13]. While promising, standardized protocols for routine clinical application of biomarkers are lacking, highlighting a key research priority [11]. Advances in molecular diagnostics and precision medicine may improve treatment outcomes and minimize unnecessary exposure to ineffective therapies.
The high cost of biologics presents a significant challenge. A cost–utility analysis conducted in the Italian healthcare setting demonstrated that dupilumab, when added to standard of care, provided an acceptable cost-effectiveness profile in patients with severe CRSwNP, supporting its use in selected, refractory cases [14,15]. Similar findings were reported by De Corso et al., who confirmed the favorable cost–utility of dupilumab within the Italian healthcare context, highlighting its potential economic sustainability when targeted to severe, uncontrolled CRSwNP [23]. Health policy must balance clinical benefit and economic sustainability, potentially using biomarker-guided patient selection to optimize resource allocation.
Table 4. Cost and Cost-Effectiveness of Treatment Options for Chronic Rhinosinusitis (CRS)
| Treatment | Cost (approximate) | Cost-effectiveness (QALYs gained) | Notes | References |
| Intranasal corticosteroids | Low | High | First-line, widely available | [8,14] |
| Macrolides | Moderate | Variable | Effective mainly in selected patients | [9,14] |
| Systemic corticosteroids | Low to moderate | Moderate | Limited long-term use due to side effects | [5,14] |
| Biologics (e.g., dupilumab) | High (several thousand USD/year) | Acceptable in selected severe cases | Cost-effectiveness depends on patient selection | [14,15] |
| ESS | Moderate to high | Variable | One-time cost, improves drug delivery | [2,10,14] |
Despite these advances, several practical and evidence gaps remain. First, patient selection is central to effective and economical use of biologics. Biomarkers including blood and tissue eosinophil counts, total IgE, periostin, and composite biomarker panels correlate with treatment response, yet consensus thresholds and standardized clinical algorithms for biomarker-guided prescribing are not universally validated [11,13,28,39]. Development and validation of simple, reproducible biomarker algorithms are priorities to optimize benefit and avoid overuse.
Second, timing and sequencing of interventions—notably the interplay between endoscopic sinus surgery (ESS) and biologic therapy—require clarification. Some evidence suggests ESS may enhance topical drug delivery and reduce inflammatory burden, potentially improving subsequent biologic responsiveness, while other data indicate early biologic initiation can reduce revision-surgery rates; pragmatic comparative studies are needed to define sequencing strategies that maximize outcomes and cost-effectiveness [2,10,16,33]. Head-to-head and pragmatic trials comparing primary biologic therapy versus ESS-first strategies would directly inform this clinical question [40].
Third, long-term safety and rare adverse events demand continued surveillance. Short-term safety profiles in pivotal trials and registries are reassuring (most commonly injection-site reactions, conjunctivitis, mild upper respiratory infections), but long-term immune modulation consequences and low-frequency events require accumulation of post-marketing registry data and pharmacovigilance studies [5,6,20,36]. National and international registries with standardized outcome and safety reporting will be indispensable.
Fourth, economic and access considerations strongly influence real-world implementation. Cost–utility and budget-impact analyses in European settings indicate that biologics can be cost-effective when targeted to patients with severe, refractory disease who have marked quality-of-life impairment or recurrent surgeries; nonetheless, reimbursement thresholds vary, and payers increasingly require biomarker-guided criteria and real-world effectiveness to support coverage decisions [14,25,35]. Health-system frameworks that integrate biomarker stratification, stepped-care algorithms, and real-world outcome tracking will better balance clinical benefit and sustainability.
Fifth, for non–type 2 phenotypes (neutrophilic CRS), evidence still supports selected use of long-term low-dose macrolides, though response rates are variable and concerns about antimicrobial resistance necessitate careful phenotype-based selection and stewardship [9,26,38]. Optimization of topical regimens—high-volume saline irrigation, targeted topical corticosteroid delivery systems and novel intranasal delivery devices—remains a cost-effective foundation across phenotypes and can potentiate systemic and biologic therapies [8,10,32].
Finally, methodological heterogeneity across studies (differences in diagnostic criteria, outcome measures, follow-up duration) complicates direct comparisons and meta-analyses. Standardization of outcome measures (NPS, SNOT-22, MCID definitions) and routine reporting of baseline endotype data will facilitate cross-study synthesis and evidence translation to guidelines [39]. Priority research areas include validated biomarker thresholds, pragmatic sequencing trials (surgery vs biologics), long-term safety registries, and head-to-head comparative effectiveness trials among biologics to inform clinical choice, guideline recommendations, and reimbursement policies [29,37].
This narrative review has several limitations inherent to its design. First, the synthesis is based on a limited number of high-quality randomized trials and real-world studies published between 2020 and 2025, which may not fully capture long-term outcomes or rare adverse events. Second, the narrative approach does not include a quantitative meta-analysis, which limits the ability to estimate pooled effect sizes and directly compare treatment efficacy across interventions. Third, although efforts were made to include current epidemiological data and recent guidelines, heterogeneity in diagnostic criteria and outcome measures among studies introduces potential bias. Fourth, levels of evidence were not formally graded, and the lack of standardized comparison of treatment strategies may reduce generalizability. Despite these constraints, the review provides an updated and comprehensive synthesis of available data on the management of acute and chronic rhinosinusitis, with particular emphasis on emerging biologic therapies and personalized approaches.
Most patients with acute rhinosinusitis require only symptomatic treatment. In chronic rhinosinusitis, regular intranasal corticosteroids and saline irrigation are the mainstay and should be optimized before systemic or surgical escalation. Antibiotics are indicated only when clear signs of bacterial infection or complications are present. Short courses of systemic corticosteroids may help in acute exacerbations. Long-term low-dose macrolides can be considered in selected non–type 2 patients who do not respond to topical therapy.
Endoscopic sinus surgery is effective when medical treatment fails and facilitates postoperative topical management. Targeted biologic therapy improves outcomes in severe CRSwNP with type 2 inflammation and reduces the need for systemic corticosteroids and revision surgery. The greatest benefit is seen in patients with persistent symptoms despite optimized conventional therapy.
Personalized strategies based on phenotype and inflammatory profile can reduce systemic exposure and repeated procedures. Limited long-term safety data, lack of validated biomarkers and unclear sequencing between surgery and biologics remain important constraints, and costs influence access.
Overall, current management combines topical therapy, surgery and biologics within individualized treatment pathways aimed at improving long-term control and quality of life.
Conceptualization: Aleksandra Rysak, Mikołaj Bluszcz, Zuzanna Muszkiet
Methodology: Aleksandra Rysak, Zuzanna Muszkiet, Michał Bar, Hubert Knapik, Damian Truchel
Investigation: Aleksandra Rysak, Zuzanna Muszkiet, Hubert Knapik, Dominik Poszwa
Data curation: Mikołaj Bluszcz, Zuzanna Dynowska, Anna Krzywda
Writing- original draft: Zuzanna Muszkiet, Mikołaj Bluszcz, Aleksandra Rysak, Dominik Poszwa, Zuzanna Dynowska, Anna Krzywda
Writing- review and editing: Zuzanna Muszkiet, Hubert Knapik, Damian Truchel, Aleksandra Rysak, Zuzanna Dynowska
Validation: Zuzanna Muszkiet, Mikołaj Bluszcz, Hubert Knapik, Dominik Poszwa
Visualization: Mikołaj Bluszcz, Damian Truchel, Aleksandra Rysak, Zuzanna Dynowska Supervision: Zuzanna Muszkiet, Mikołaj Bluszcz, Damian Truchel, Anna Krzywda
Project administration: Zuzanna Muszkiet, Michał Bar, Damian Truchel, Dominik Poszwa
The authors acknowledge the use of artificial intelligence tools, Grok (created by xAI) and ChatGPT (created by OpenAI), for assistance in drafting initial versions of certain sections and polishing the language of the manuscript. These tools were used to enhance clarity and organization of the text. All content was thoroughly reviewed, edited, and validated by the authors to ensure scientific accuracy, integrity, and alignment with the study’s objectives.
The authors declare no conflicts of interest.