- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2023. 13; 6: e1. DOI 10.35630/2023/13/6.602
Purpose of the Study: To analyze the effectiveness of using sodium deoxyribonucleate (SDN) for pelvic adhesions in rats in experiment.
Materials and Methods: The experiment was carried out on 90 laboratory rats. Three groups of 30 animals each were formed: group 1 – control; group 2 – rats with a simulated adhesive process in the pelvis without subsequent treatment; group 3 – animals with a model of adhesive process in the pelvis receiving intramuscular SDN for 10 days. The adhesive process in the pelvis was modeled in rats by ligating the uterine horns on both sides. The expression of CD4, CD8, CD20, CD138 was studied using DP-SOFT software program and subsequent statistical processing.
Results: In animals of the control group, a small number of cells with expression of CD4+, CD8+ and CD138 markers were found in the parietal peritoneum (PP). In perimetrium (PM), no positively stained cells of the studied immunophenotype were found. In group 2, animals showed an increase in the number of cells in the PP positively expressing CD4+ and CD138+, and the appearance of CD56+ cells. An increase in the number of CD138+ and CD4+ cells was found in the rats’ PM. In group 3, compared to the control, the count of CD4+ cells, CD56+ cells and CD138+ cells significantly increased. In the PM of the operated animals, we found an increase in the number of cells positively expressing CD4+ and CD138+, and the appearance of CD56+ cells. In the PM of rats, an increase in the number of CD138+ and CD4+ cells was noted, which indicates activation of the humoral immune system. In group 3, the count of CD4+, CD56+ and CD138+ – cells in the PP significantly increased compared to the control. PM was characterized by a similar distribution of cells.
Conclusion: After surgery, the adhesive process causes a change in the ratio of leukocyte subpopulations in the affected area towards an increase in their number in the parietal peritoneum rather than in the perimetrium. The use of SDN has an immunomodulatory effect and stimulates immune responses by increasing the number of immunocompetent cells in the surgical site.
Keywords: adhesions, pelvic adhesions, surgery, immunocytochemistry, markers, prevention, sodium deoxyribonucleate
Intra-abdominal adhesions are a special form of internal scar tissue that occurs as a result of damage to the peritoneum – a membrane that lines all organs of the abdominal cavity. This scar tissue can cause organs to stick to each other or to the abdominal wall. The vast majority of adhesions occur after surgery, but inflammation and radiation therapy can also cause adhesions. Adhesions develop both after open abdominal surgery (in 90% of patients) and after laparoscopic interventions (in 70%). Adhesions after laparoscopy are often less extensive. Although most adhesions are asymptomatic, they represent a significant social burden of disease. This is partly due to the fact that half of the total population will undergo abdominal surgery during their lifetime [1-6].
The four main consequences of adhesions are: lifelong risk of intestinal obstruction, chronic pain, increased risk of iatrogenic injury during reoperation, and female infertility. Acute adhesive intestinal obstruction is an acute condition in which the intestines become closed by adhesions, resulting in cramping abdominal pain, nausea and bloating. After abdominal surgery, up to 20% of patients complain of chronic pain, perhaps half of which are caused by adhesions. Reoperations are associated with an average of at least 15 minutes of additional operative time due to the need for adhesiolysis. It also greatly increases the risk of iatrogenic injury to the bowel, bladder, or other organs. Adhesions around the internal genital organs are the cause of decreased fertility in 3 out of 10 women with acquired infertility [7-13].
Several agents have been developed to prevent adhesions, such as antiadhesive coatings, anti-inflammatory and fibrinolytic drugs. The Food and Drug Administration (FDA) has approved the use of physical barrier agents, but they have been associated with contradictory clinical studies and diverging opinions on the clinical use of antiadhesive barriers. Release agents are biodegradable substances that remain in the abdominal cavity after surgery. These agents separate the wound surfaces from each other, making them less likely to adhere. Adhesion barriers are available in various forms such as liquids, gels or membranes. Such barriers are still relatively little used due to the side effects and complications caused by some of these drugs. As a result of systematic clinical studies, several antiadhesive drugs currently available on the market have been found to be safe and effective for their indications. The most commonly used barriers in abdominal surgery are a membrane containing hyaluronic acid and fluid (Adept, with 4%). In gynecology, barriers in the form of a gel are also used, for example, hyalobarrier (cross-linked hyaluronic acid). These gel barriers are easier to use for topical laparoscopic use, but have not been tested for safety in intestinal surgery with associated contamination. The membrane and fluid can be used in intestinal surgery. In the presence of a membrane, it is not recommended to apply the film directly to a fresh intestinal suture. The fluid is safe in combination with intestinal sutures, but it interferes with postoperative CT imaging due to the large amount of free fluid. Therefore, surgeons are somewhat wary of using these products on intestinal sutures, especially in sutures with an increased risk of leakage or in cases of massive contamination [14-19].
In addition to surgical, there are also pharmacological methods - the use of non-steroidal and steroidal anti-inflammatory drugs, fibrinolytics, immunomodulators, antitumor drugs and cellular technologies. At the present time, in-depth study of the pathophysiology of the adhesive process has shown its multifactorial nature, and the role of cellular and molecular modulators of adhesion, which makes it possible to develop new types of preventive effects on adhesions. Until now, there is no consensus on the reaction of local lymphoid elements during the formation of peritoneal adhesions. The significance of tissue immunological changes in excessive adhesiogenesis in inflammatory diseases and after surgical interventions on the abdominal organs has not been determined [20-23].
The purpose of the study is to evaluate the capabilities of sodium deoxyribonucleate (SDN) in modulating the reactions of immunocompetent cells of the parietal peritoneum and perimetrium for abdominal adhesions in experimental rats.
The experiment was carried out on 90 laboratory animals - white non-linear rats with an average weight of 280 ± 30 g., kept under normal vivarium conditions. Animal studies were carried out in accordance with the international rules of “Guide for the Care and Use of Laboratory Animals” with free access to combined food and water. Three groups of 30 animals each were formed: 1 group – control, which served as the norm for the studied indicators; group 2 – rats had a simulated adhesive process in the pelvis and did not receive any treatment; group 3 – animals with a model of postoperative pelvic adhesions were administered with SDN 1.5% intramuscularly in a volume of 1.0 ml for 10 days. The adhesive process in the pelvis was modeled under anesthesia by ligating the uterine horns on both sides at a rate of 100 g. animal body weight. The collection of adhesions was carried out on the day 28 after modeling the adhesive process, i.e., in the phase of mature adhesions.
Immunohistochemical examination of adhesive tissue was carried out according to a standardized method using serial paraffin sections 3-5 µm thick, placed on polysine-coated adhesive glasses (Menzel-Glaser, Germany) and DAKO reagents with monoclonal mouse antibodies CD4 (Clone 4B12 Ready-to-Use), CD8 (Clone C8/144B Ready-to-Use), CD20 (Clone L26 Ready-to-Use), CD 138/syndecan-1 (Clone MI15 Ready-to-Use) and NovocastraNovolinkTM imaging systems based on compact Novolink Compact Polymer™ (Leica, Germany) on a BondMax immunohistostainer (Leica, Germany). The standard procedure included the use of negative and positive controls.
The morphometric study included measuring the number of positively stained cells in 10 randomly selected fields of view at a magnification of ×400 with immunohistochemical staining with lymphocyte markers CD4, CD8, CD20, CD138 using the Software DP-SOFT program and subsequent statistical processing.
The microslides were viewed and photographed with an OLYMPUS C 5050Z digital camera mounted on an Olympus CX-41 microscope.
Statistical data was processed using the application package “Statistica for Windows” v. 7.0, StatSoft Inc. Data were checked for normal distribution using the Shapiro-Wilk criteria.
The values to be analyzed were assessed by calculating the arithmetic mean (X) and its standard deviation (SD). Comparisons between groups were made using nonparametric methods using the Mann-Whitney test. Differences were considered statistically significant at p<0.05 (95% significance level) and at p<0.01 (99% significance level).
In animals of the control group, a small number of cells with expression of CD4+ (1.20 per field of view), CD8+ (4.00 per field of view) and CD138+ (5.90 per field of view) markers were found in the parietal peritoneum (PP), located next to the vessels of the hemomicrocirculatory bed and mesothelium. CD138+ (syndecan-1) is a highly specific marker of plasma cells, a membrane proteoglycan that functions as a receptor for the extracellular matrix and is also present on the surface of endothelial cells. Cells expressing CD138+ are present in the PP, providing a background level of serum IgM (0.6 - 3.7 g/l).
Considering that CD8+ is a transmembrane protein, a co-receptor of T lymphocytes and is expressed on suppressor T lymphocytes, it seems logical that cells with this immunophenotype would be present in the parietal peritoneum, where they can control (inhibit) the production of antibodies (of various classes) due to delays in proliferation and differentiation of B-lymphocytes into plasmacytes. The dominance of CD8+ expression over that of CD4+ (4.00/1.20) in the control group indicates the absence of antigenic load and inhibition of cellular and humoral immunity reactions (Table 1).
Table 1 The immunophenotypic characteristics of lymphoid cells distribution in the parietal peritoneum in the control groups (number of cells in the field of view, X ±SD)
| Cell phenotype | Parietal peritoneum |
| CD4+ | 1,20±0,13 |
| CD8+ | 4,00±0,10 |
| CD56+ | not found |
| CD138+ | 5,90±0,14 |
In the perimetrium (PM) of animals in the control group, no positively stained cells of the studied immunophenotype were found, except for single cells expressing CD138+ (the average number of cells in the field of view was 5.9), which most likely formed the effector link of immune memory.
After surgery, an increase in the number of cells positively expressing CD4+ and CD138+ and the appearance of CD56+ cells were detected in the parietal peritoneum of animals, which resulted in arranging the expressed markers in the following order (according to the number of positively labeled cells): CD138+ > CD8+ > CD4+ > CD56+. A twofold increase in CD4+ cells (probably Th1) against the background of a slightly reduced number of CD8+ cells compared to the control in this case may be accompanied by a stimulating effect on fibroblasts and fibrillogenesis, promoting adhesions. Against the background of the appearance of single cells positively expressing CD56+ in the rats’ parietal peritoneum, we found an increase in the number of CD138 cells to 10.00 per field of view and CD4+ cells to 2.55 per field of view, which indicates activation of the humoral component of immune reactions. The increase in the number of CD138+ compared to controls may reflect increased migration of plasma cells leaving lymphoid follicles into connective tissues, which often arise as a result of differentiation of B-2 cells along the extrafollicular pathway. Table 2.
Table 2. The immunophenotypic characteristics of lymphoid cells distribution in the parietal peritoneum after surgery and treatment with SDN
| Cell phenotype | М number of cells in the field of view, X ±SD | Mann-Whitney U test | Statistical Significance Level | |
| Without treatment | SDN treatment | |||
| CD4+ | 2,55±0,15 | 9,55±0,12 | 290 | p=0,008 |
| CD8+ | 3,55±0,13 | 2,55±0,13 | 594 | p=0,133 |
| CD56+ | 1,80±0,14 | 5,20±0,10 | 742 | p=0,505 |
| CD138+ | 10,00±0,16 | 13,00±0,11 | 311 | p=0,036 |
In the group of animals that received SDN in the postoperative period, compared to the control, there was a significant increase in the count of CD4+ cells (7.95 times), CD56+ cells (5.20 per field of view) and CD138+ cells (2.2 times). The quantitative series of cells expressing the studied markers took the sequence: CD138+ > CD4+ > CD56+ > CD8+. These changes in the cellular composition of the parietal peritoneum should be interpreted as activation of cellular and humoral immunity. Table 3.
Table 3. The immunophenotypic characteristics of lymphoid cells distribution in the perimetrium after surgery and treatment with SDN
| Cell phenotype | М number of cells in the field of view, X ±SD | Mann-Whitney U test | Statistical Significance Level | |
| Without treatment | SDN treatment | |||
| CD4+ | 2,75±0,11 | 8,00±0,14 | 173 | p=0,008 |
| CD8+ | 2,20±0,10 | 1,70±0,18 | 619 | p=0,861 |
| CD56+ | 2,10±0,13 | 4,10±0,12 | 307 | p=0,022 |
| CD138+ | 8,90±0,12 | 11,25±0,15 | 226 | p=0,007 |
In the perimetrium of the operated animals, the expressed markers took the sequence (based on the number of positively labeled cells): CD138+ > CD4+ > CD8+ > CD56+. We noted an increase in the number of cells positively expressing CD4+ and CD138+, and the appearance of CD56+ cells. Fig. 1.
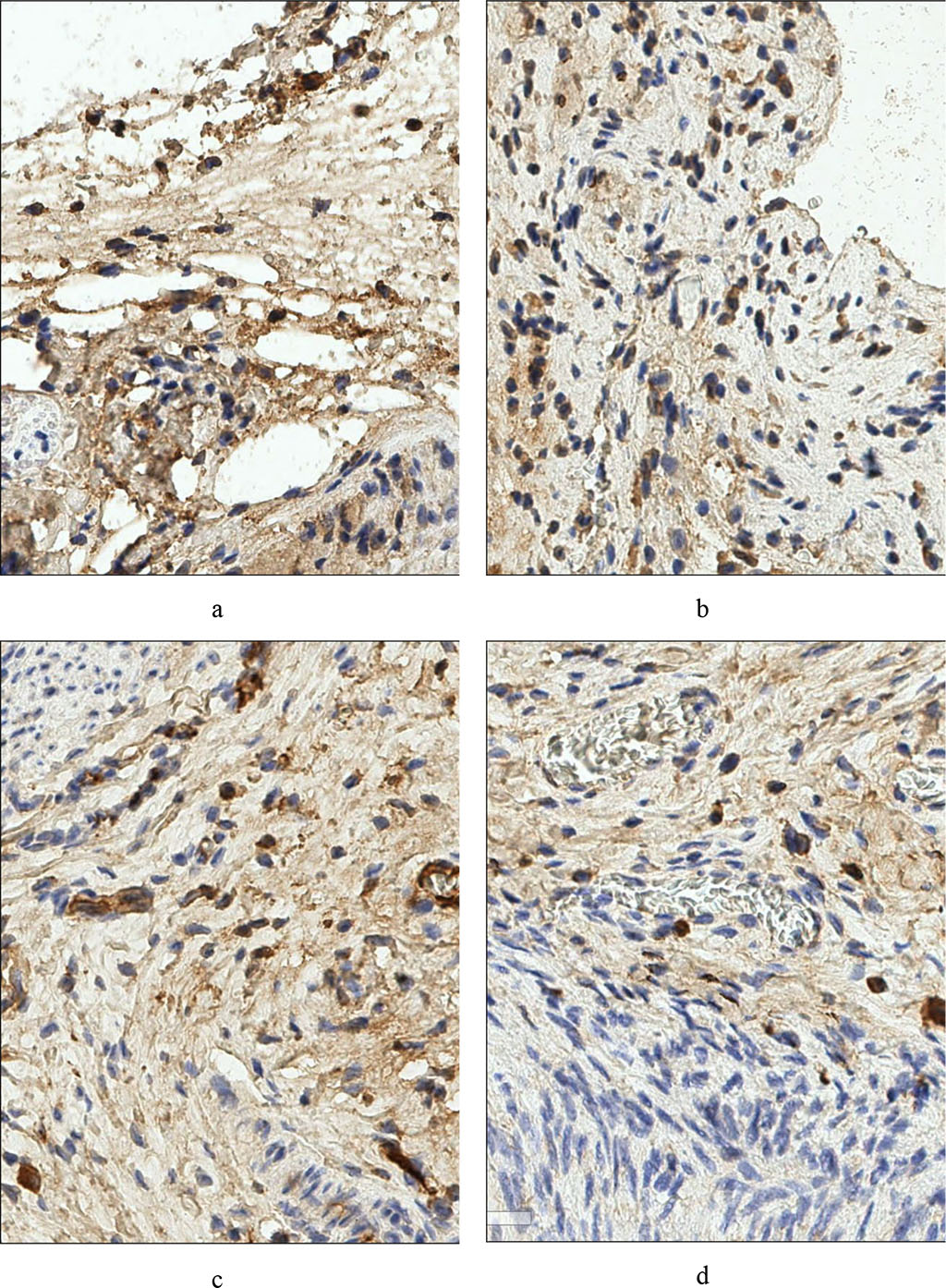
Fig. 1 – Rat's peritoneum. Expression of the marker CD4+ a - before treatment, b - after treatment. Rat parametry. Expression of CD138+ c marker - pre-treatment, d – post-treatment (×400)
Against the background of the appearance of single cells positively expressing CD56+ in the rats’ perimetrium, we found an increase in the average number of CD138+ cells to 8.90 per field of view and CD4+ cells to 2.55 per field of view, which indicates activation of the humoral component of immune reactions. The quantitative series of cells expressing the studied markers took the sequence: CD138+ > CD4+ > CD8+ > CD56+. However, the CD8+ population was not statistically significantly different from that before surgery.
In the group of animals that received SDN in the postoperative period, there was a significant increase in the count of CD4+ cells (7.95 times), CD56+ cells (5.20 per field of view) and CD138+ cells (2.2 times) in the PP compared to the control (Fig. 2). Based on the number of positively labeled cells, the expressed markers took the sequence CD138+ > CD56+ > CD4+ > CD8+. The perimetrium was characterized by a similar distribution of cells in the quantitative series: CD138+ > CD56+ > CD4+ > CD8+, which was due to an increase in the number of CD56+ cells (11.25 in the field of view), CD4+ cells (6.67 times compared to the control) and CD56+ cells (2 times compared to those without SDN treatment). These changes in the cellular composition of the PM should be interpreted as activation of cellular and humoral immunity, similar to that in the PP. Fig. 2.
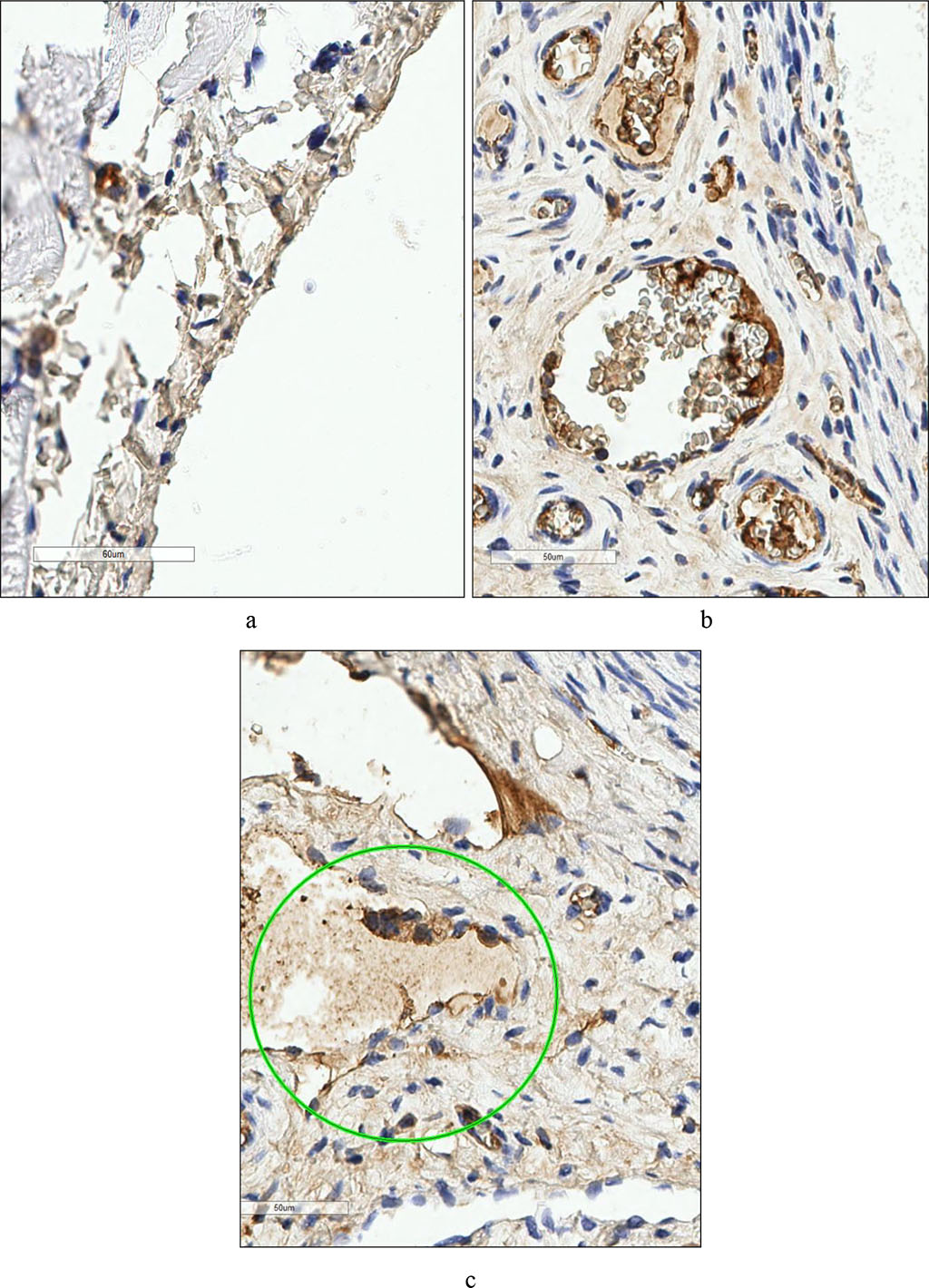
Fig. 2. Rat's peritoneum. Expression of the marker CD56+ a - in the control group, b - after surgery. Rat parametry. Expression of CD56+ c marker - post-treatment. (×400).
Noteworthy was the similar reaction of various phenotypes of immunocompetent cells in the parietal peritoneum and perimetrium when using SDN in the postoperative period. In both cases, the greatest degree of increase was observed in the number of CD4+. Judging by the increasing dynamics of the number of CD56+ and CD138+, we can assume the dominance of the Th2 phenotype CD4+, which secretes interleukins 4, 5, 10, 13 and thus regulates the proliferation and differentiation of other phenotype lymphocytes. Interleukin 10 is produced not only by T helper cells type 1 and 2 (Th1 and Th2), but also by macrophages and cytotoxic cells (CD56+), exerting a pronounced immunosuppressive effect, reducing the proliferative activity of T cells and the functional activity of monocytes/macrophages. The immunosuppressive effect of the cytokine apparently compensates for the decrease in the number of CD8+ phenotype cells in rats of both experimental groups compared to the control one. At the same time, interleukin 10 can play the role of a factor stimulating CD56+ differentiation, which is manifested by a significant increase in cells of this phenotype in rats treated with SDN in the postoperative period. The shift in CD4+ differentiation towards Th2 may be reflected in an increase in the count of CD138+ cells, the proliferation cofactor of which is interleukin 4 secreted by Th2 cells. CD138+ are plasma cells formed either in response to TI antigens (descendants of B-1 cells), or as a result of differentiation of B-2 cells along the extrafollicular pathway. The latter are capable of developing under the influence of cellular microenvironmental factors (autoaggressive clones of T-lymphocytes expressing CD40, or Th2, secreting interleukins 4 and 5) [24].
Thus, judging by the expression of immunocytochemical markers in the control group of rats, there are differences in the cellular composition of leukocytes in the parietal peritoneum and perimetrium. After surgery, the adhesive process causes a change in the ratio of leukocyte subpopulations in the affected area towards an increase in the number of CD4+, CD56+ and CD138+ cells, in the parietal peritoneum rather than in the perimetrium. The use of SDN has an immunomodulatory effect by redistributing the number of various phenotypes of immunocompetent cells in the connective tissues of the pelvis, which may be due to changes in the secretion of regulatory cytokines 4 and 10. Establishing the role of various phenotypes of lymphocytes of connective-tissue post-operative adhesions in the regulation of local immune status through the secretion of cytokines requires further research.