- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.9
This study addresses the influence of the ethanol content in the body on the development of fatal hypothermia. All cases were divided into 3 groups. The first group included 590 cases in which glycogen in the liver, skeletal muscle and myocardium was not determined. The second group consisted of 540 corpses of people who had glycogen detected in the liver, in different concentrations, and its absence in the skeletal muscle and myocardium. The third group of the study showed a sharp decrease in the content of glycogen or its absence in the skeletal muscle with its reduction in other tissues – 580 cases. There were 72,2%, – 67,8%, – 51,2% of the dead in the state of alcoholic intoxication in the studied groups, respectively. The ethanoluria/ethanolemia ratio was significantly decreased with the increase of ethanol in the blood. Thus, the use of ethanol is a risk factor for fatal hypothermia, contributing to faster utilization of glycogen in the tissues, which reduces the survival time at low ambient temperatures. The use of biochemical analysis by the content of glycogen in the tissues allows differential diagnosis of the immediate cause of death due to hypothermia in the presence of high and toxic concentrations of alcohol in the body.
Keywords: Hypothermia, ethanol, glycogen.
The effect of extreme temperatures takes the fourth place among the causes of death, while the proportion of hypothermia is about 80% [1]. In the general structure of mortality of the population, cases of fatal hypothermia range from 3.5 to 11%, depending on the region [2, 3]. One of the criteria for thanatogenesis due to hypothermia is a sharp decrease or absence of glycogen in the liver, skeletal muscle and myocardium. These indicators reflect the metabolic disorder, as the result of the depletion of regulatory and compensatory mechanisms in ensuring the energy production of the body to maintain temperature homeostasis. Biochemical studies for the determination of glycogen in tissues are used in laboratory practice for hypothermia diagnosis [4-7] and post-mortem differential diagnosis of the cause of death [7]. Our previous studies showed that the content of glycogen and lactate in skeletal muscle was the most informative and reliable indicator for the hypothermia diagnosis [8]. The risk factor contributing to fatal hypothermia is ethanol consumption. The proportion of those who died from lethal hypothermia while under the influence of alcohol is 36–87% [2, 9, 10]. The influence of ethanol on the development of lethal hypothermia was studied in animals by numerous authors in the 1950s and 1970s, with conflicting data obtained. Most studies have confirmed that alcohol contributes to the development of hypothermia by lowering body temperature; some studies have indicated that the body is resistant at low temperatures [10].
The purpose of the study was to study the effect of ethanol content in the body on the development of lethal hypothermia.
The objects of the study were the tissues of the liver, skeletal muscle, myocardium, blood, urine of people who died as a result of hypothermia. The diagnosis was based on a complex of morphological, histological and biochemical studies. For statistical analysis, all deaths due to hypothermia were divided into 3 groups. The first group included 590 cases in which glycogen in the liver, skeletal muscle and myocardium was not determined. The second group consisted of 540 corpses of people who had glycogen detected in the liver, in different concentrations, and its absence in the skeletal muscle and myocardium. The third group of the study showed a sharp decrease in the content of glycogen or its absence in the skeletal muscle with its reduction in other tissues – 580 cases.
Determination of glycogen content in tissues was carried out in accordance with the method developed by us [11, 12]. The method principle is to pre-fix the tissue in acetone, followed by sample preparation by homogenizing the biological tissue and carrying out the acid hydrolysis of glycogen to glucose, followed by the determination of glucose level using the enzymatic glucose oxidase method. The proposed method makes it possible to study biological objects within a long period after material sampling, while the quantitative result does not depend on the time between object sampling and the laboratory study. The content of ethanol in blood and urine was determined by a modified alkyl nitrite gas chromatographic method on a Kristall-2000 chromatograph [12].
In the first group of the dead, ethanol was found in the blood and urine in 72.2%, in the second group – 67.8%, in the third group – 51.2%, which corresponds to the literature data. It is known that there are stages of resorption and elimination in the toxicokinetics of ethanol. Upon alcohol ingestion, ethanol concentration in the blood increases rapidly, and decreases gradually. The maximum concentration of ethanol in the blood in most people is observed approximately one hour after alcohol ingestion (from 45 minutes, if alcohol is ingested on an empty stomach, up to 3 hours – depending on the nature of the food) [13, 14].
There is evidence that people who died from hypothermia often have low ethanol concentration in the blood, although it is known from the facts of the case about ingestion of large amounts of ethyl alcohol shortly before the incident [13]. The authors attributed this to the rapid utilization of ethanol.
According to our data, death as a result of hypothermia is observed in most cases at the stage of elimination, when ethanol is already eliminating out of the body. Only in 4.3% of cases, lethal hypothermia affected by alcohol intoxication progressed to the stage of resorption, regardless of the level of ethanol in the blood (Fig. 1). In these observations, a fairly rapid depletion of glycogen indicates a rapid rate of hypothermia of the body and is most likely associated with an initially low glycogen level in tissues.
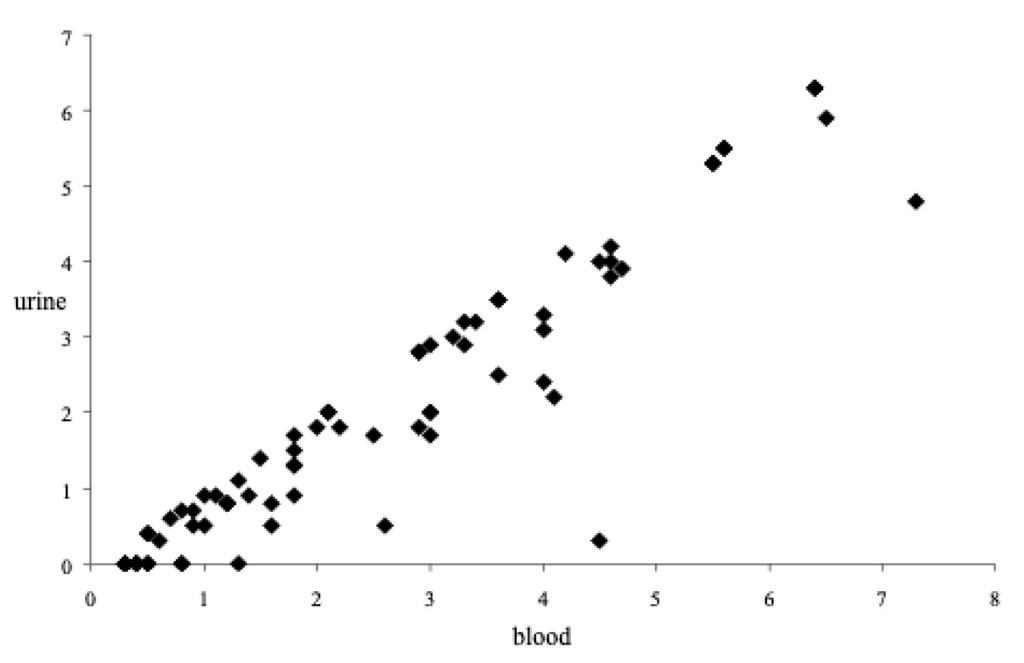
Figure 1. Ethanoluria/ethanolemia ratio in the ethanol resorption stage
In the study of cases of hypothermia, the ratio of ethanol level in the urine to ethanol level in the blood (ethanoluria/ethanolemia) was studied. An inversely proportional dependence of this indicator in all three groups was detected. A significant decrease in the ratio with an increase in the level of ethanol in the blood was established (Table 1). For the analysis of the ethanoluria/ethanolemia ratio, cases of the presence of ethanol only in the blood or only in the urine (weak concentrations) were excluded. This ratio is an indicator of the ethanol metabolism dynamics in the body. Such changes indicate a more rapid death at high concentrations of ethanol in the body. Complete depletion of glycogen indicates a slow rate of hypothermia.
Table 1 – Distribution of the ethanoluria/ethanolemia ratio by the content of ethanol in the blood during hypothermia
| Ethanol content (‰) in the blood | n1 |
Ethanoluria/ ethanolemia ratio | n2 |
Ethanoluria/ ethanolemia ratio | n3 |
Ethanoluria/ ethanolemia ratio |
| Less than 0.5 | 28 | 2.26 ± 0.28 | 26 | 2.81 ± 0.74 | 8 | 2.61 ± 0.59 |
| 0.6 – 1.0 | 53 | 2.15 ± 0.13 | 26 | 1.79 ± 0.18 | 26 | 1.76 ± 0.18 |
| 1.1 – 1.5 | 76 | 1.89 ± 0.06 | 47 | 1.69 ± 0.07 | 34 | 1.57 ± 0.08 |
| 1.6 – 2.0 | 81 | 1.74 ± 0.05 | 70 | 1.63 ± 0.05 | 36 | 1.63 ± 0.09 |
| 2.1 – 2.5 | 69 | 1.65 ± 0.04 | 69 | 1.70 ± 0.05 | 46 | 1.61 ± 0.07 |
| 2.6 – 3.0 | 50 | 1.53 ± 0.04 | 49 | 1.70 ± 0.26 | 37 | 1.47 ± 0.07 |
| 3.1 – 3.5 | 32 | 1.38 ± 0.03 | 32 | 1.42 ± 0.05 | 21 | 1.37 ± 0.05 |
| 3.6 – 4.0 | 16 | 1.28 ± 0.08 | 20 | 1.45 ± 0.12 | 15 | 1.45 ± 0.16 |
| 4.1 – 4.5 | 11 | 1.22 ± 0.11 | 11 | 1.13 ± 0.04 | 9 | 1.15 ± 0.15 |
| 4.6 – 5.0 | 7 | 1.11 ± 0.07 | 10 | 1.15 ± 0.07 | 4 | 1.13 ± 0.12 |
| More than 5.0 | 3 | 0.91 ± 0.14 | 6 | 1.06 ± 0.05 | 6 | 1.03 ± 0.04 |
| n1
– group 1
n2 – group 2 n3 – group 3 |
||||||
Cases with toxic content of ethanol in the body were analyzed. For this, the highest concentration of alcohol in the urine was taken into account. The data obtained are presented below (Table 2). The largest number of deaths with toxic concentrations was noted in the first group – with complete utilization of glycogen in all tissues, the smallest number – in the third group – when glycogen was not fully used by the body. Thus, every fifth person who died as a result of hypothermia was in a state of severe alcohol intoxication.
Table 2. Proportion of deaths due to fatal hypothermia in the presence of toxic concentrations of ethanol in the body
| Group | 4.1 – 5.0 ‰ | 5.1 – 6.0 ‰ | over 6.0 ‰ | Total |
| 1 | 15.6% | 4.1% | 1.5% | 21.2% |
| 2 | 12.6% | 5.7% | 2.0% | 20.4% |
| 3 | 7.1% | 3.6% | 2.4% | 13.1% |
| Total | 11.8% | 4.4% | 2.0% | 18.2% |
Based on the data obtained, it can be concluded that the greater the concentration of ethanol in the body, the less time has passed from the beginning of the elimination stage to the time of death. Toxic concentrations of alcohol contribute to faster glycogenolysis in skeletal muscle. It is known that the oxidation of alcohol in the body is followed by gluconeogenesis inhibition and glycogenolysis activation [15, 16] in the liver. At the same time, with lethal hypothermia, in some cases, a significant amount of glycogen in the liver remains, for example, in the second group, a slight or moderate decrease in glycogen was noted in 15.9% of cases.
Thus, the obtained results confirm the fact that the use of ethanol is a risk factor in fatal hypothermia that contributes to faster utilization of glycogen in tissues, which reduces the survival time at low ambient temperatures.
The use of biochemical analysis by the content of glycogen in tissues allows differential diagnosis of the immediate cause of death as a result of hypothermia in the presence of high and toxic concentrations of alcohol in the body.