- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.6
In order to study the features of the development of the basilar artery, the authors analyzed 4 series of sagittal sections and 2 series of frontal sections of human embryos from 21 mm to 27 mm crown-rump length. The primary basilar artery is detected at 7 weeks of fetal development. It turned out that in embryos of 7–8 weeks of human prenatal ontogenesis, the arteries already vascularize the brain well, and the arterial bed of the human brain can be ready for functioning. The data obtained indicate the emergence of the irrigation system of the basilar artery at the age of 8 weeks of intrauterine development in humans. The analyzed stages of embryos are assumed as a kind of spurt for the development of the arterial system of the human brain and determine the need for further fundamental longitudinal study of human embryogenesis.
Keywords: human basilar artery, human embryogenesis, cerebral arteries, prenatal ontogenesis, development of cerebral arteries
Further in-depth study of the regularities of ontogenesis will make it possible to explain the origin of variants and abnormalities in the organism development: congenital abnormalities and deformities are deeply rooted in the processes of early embryogenesis [1–5].
To date, the concept of the emergence and development of cerebral arteries in the human embryonic period is as follows. In weeks 3–4 of fetal development, the internal carotid arteries are formed by the third pair of branchial arches on each side or by anastomosis of the corresponding dorsal aorta and the third pair of branchial arches. The internal carotid arteries on the left and right are formed directly according to the type of plexiform formation. In weeks 3–4 of fetal development, branches (including the persistent trigeminal artery) are outlined from the primary internal carotid arteries. By the 4th week of intrauterine development, paired primary basilar arteries (treated as the irregular basilar artery) are formed, connected to the internal carotid arteries through persistent trigeminal arteries. In weeks 4–5 of fetal development, primary vertebral arteries are formed dorsally from the dorsal aortas from the cervical branches of the intersegmental arteries. In weeks 5–6 of fetal development, the primary vertebral arteries are involved in the formation of an integral primary basilar artery. In weeks 5–8 of fetal development, anastomoses are outlined by the posterior communicating arteries with the primary branches of the internal carotid arteries, thus forming the arterial circle of the brain. In weeks 5–8 of fetal development, the superior cerebellar and inferior anterior cerebellar arteries emerge from the primary basilar arteries, and the posterior inferior cerebellar arteries emerge from the primary vertebral arteries [6–12].
However, all the studies cited generally indicate that the ontogenesis of the arterial bed of the human brain has not been studied enough. Fundamental manuals on human embryology are sketchy. In addition, there is no systematic data on the development of cerebral arteries, in light of the ever-increasing need to study the formation of variants, abnormalities and congenital malformations of cerebral vessels.
To identify the features of the formation of the human basilar artery during embryogenesis, we analyzed 4 series of sagittal sections and 2 series of frontal sections of human embryos from 21 mm to 27 mm crown-rump length, stained with hematoxylin-eosin.
On the series of sections of human embryos, we studied the rudiments of parts of the nervous system in conjunction with the anlages of the arteries supplying the brain. The study of the material was carried out on a large universal light microscope "NU" (Germany), eyepiece x12.5; lenses x10, x25, x63, x100, and a Leica MZ 12.5 stereo microscope using a Pixera television color camera (USA) and the Photo Shop program.
Embryos of 21 mm crown-rump length (CRL) (7 weeks of intrauterine development) clearly show the endbrain, interbrain, midbrain, hindbrain and spinal cord. The anlage of the endbrain contains an irregularly elongated cavity, while in the anlages of the underlying parts of the brain no cavitary formations are found. In these parts of the brain, the anlages of future arteries and arterial plexuses are determined. However, the arterial plexus anlages in the endbrain are less clear. In the anlages of the hindbrain and spinal cord, 1–2 large arteries are identified. Caudally from the endbrain and ventrally from the midbrain, but in the interbrain, the anlages of the eye cup and eye are determined. Dorsally from the eye cup, on the border of the midbrain and interbrain, the anlage of the spinal ganglion is clearly identified, with nerve trunks extending from it to the endbrain. Embryos of 7 weeks of intrauterine development have anlages of clearly defined both primary internal carotid arteries, anlages of persistent trigeminal arteries, anlages of primary vertebral arteries, anlages of the primary basilar artery (Fig.).
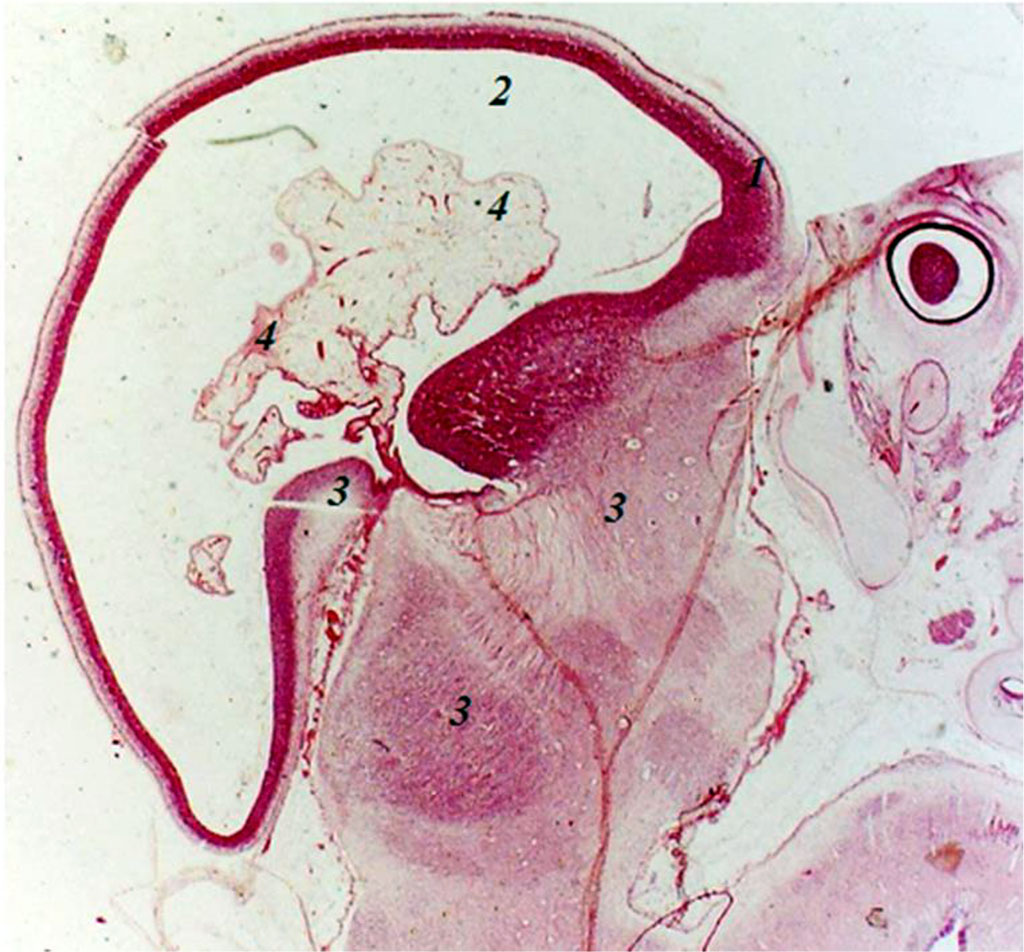
Fig. Sagittal section of the embryo of 8 weeks of intrauterine development: 1 - endbrain wall; 2 - endbrain cavity; 3 - anlages of arterial trunks; 4 - plexiform branching of arterial anlages; 5 – anlage of the eye cup. Increased by 160.
In embryos of 27 mm CRL (8 weeks of intrauterine development), the anlage of the endbrain contains an irregularly elongated cavity. In the cavity and walls (there are 3 of them) of the endbrain anlage, the anlages of the arteries are found, sometimes the anlage of the artery with elements of plexiformity is clearly detected. The cavity of the endbrain is closely adjacent to the underlying sections and that’s where the anlage of the arterial system derives from. Moreover, the anlage of this plexus is formed from the anlages of the arteries lying between the anlages of the endbrain and the underlying parts of the brain, where the anlages of the arteries are also detected. In the caudal-ventral part of the hindbrain, a cavity with two walls is revealed, with arterial anlages in its lumen. Embryos of 8 weeks of intrauterine development have anlages of both primary internal carotid arteries with an emerging plexus of anlages of branches of the primary internal carotid arteries, anlages of the primary vertebral arteries, anlages of the primary basilar artery with plexiform branching.
Thus, at the age of 7–8 weeks of intrauterine development, 1–2 large arterial trunks are detected in the anlages of the hindbrain and spinal cord. At the age of 8 weeks of intrauterine development, plexiform anlages of the arterial system are found in the anlages of the endbrain, and anlages of arteries are detected in the caudoventral part of the hindbrain.
Consequently, the materials of this section of research show that the primary basilar artery is detected at 7 weeks of intrauterine development, and in embryos of 8 weeks of human prenatal ontogenesis, the arteries are likely to vascularize the brain sufficiently for age needs.
The above circumstances allow us to suggest the analyzed developmental stages as a kind of spurt for the arterial system of the brain and determine the need for further fundamental study of the arterial bed of the human brain in the ontogenetic aspect, which is consistent with the data from available sources [13-15].