- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.5
The aim of the study was to evaluate behavioral disorders in experimental metabolic syndrome and the possibility of treatment with complex phytoadaptogens (CPhA). The experiment was carried out on 30 male Wistar rats randomly divided into 3 groups: control (Group 1), metabolic syndrome (MS, Group 2), and treatment of metabolic syndrome with CPhA (Group 3). In Groups 2 and 3, the animals were on a diet high in carbohydrates and fats for 16 weeks. Group 3 animals received CPhA for 14 days with drinking water after 16 weeks of the diet. CPhA consist of standard tinctures of Glycyrrhiza glabra, Rhodiola rosea, Acantopanax senticosus in a ratio of 1:2:1. Behavior was analyzed in the open field test (OFT) and the elevated plus maze (EPM), using Realtimer software (OpenScience, Russia). Data were analyzed using GrafPadPrism 8.03 software (USA). The experiment demonstrated that metabolic syndrome is associated with increased anxiety (decrease in horizontal (p=0.017) and vertical (p=0.017) locomotor activity) and fear (increase in immobility time (p=0.011)) in the OFT. Increased anxiety of animals (decreased open arm time (p=0.012) and increased closed arm time (p=0.043)) and emotional stress (increased frequency of defecation (0.017)) relative to control are also confirmed by EPM data. The data obtained in the treatment group (no significant differences with the control), i.e., a decrease in the manifestations of fear and anxiety (increased orientation and exploratory activity), indicate that the complex phytoadaptogens are an effective anxiolytic. The mechanisms that led to this result remain to be explored, highlighting the role of the autonomic nervous system, leptin and ghrelin in behavior and the effect of the complex phytoadaptogens on them.
Keywords: Acanthopanax senticosus; dyslipidemia; Glycyrrhiza glabra; hypertension; insulin resistance, metabolic syndrome; obesity; Rhodiola rosea
The prevalence of metabolic syndrome (MS) is becoming a serious medical and social problem worldwide. According to the WHO, more than 1.9 billion people are overweight [1, 2]. MS is characterized by insulin resistance, hyperglycemia, dyslipidemia, hypertension, and obesity, and is a proinflammatory and prothrombotic state. The main role in the development of MS is played by adipose tissue, a biologically active endocrine and paracrine organ. It produces adipocytokines, which include the main pro-inflammatory mediators interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), and plasminogen activator inhibitor-1 (PAI-1). Cytokines induce an inflammatory response and decrease the sensitivity of insulin receptors. Obesity progresses rapidly with the development of metabolic disorders; increases the risk of developing type 2 diabetes and cardiovascular disease. In recent years, there has been a growing interest in studying the relationship between MS and psychosomatic disorders, such as depression and anxiety. Study results are controversial, but it has been shown that pro-inflammatory cytokines cause depressive disorders and that depression itself induces eating disorders leading to MS, exhibiting pro-inflammatory properties [3, 4].
In this study, we explored a new herbal formula that can be effectively used in MS and obesity, a phytococktail consisting of well-known and widely used phytoadaptogens: Glycyrrhiza glabra, Rhodiola rosea, Acanthopanax senticosus. Rhodiola rosea and Glycyrrhiza glabra have anti-inflammatory and antioxidant effects. Acanthopanax senticosus regulates homeostatic responses through the neuroendocrine immune (NEIM) system. They control stress-activated molecular chaperones (Hsp70), cortisol, and nitric oxide (NO). Under stressful conditions, the adaptogens modulate pineal gland function [5]. In addition, according to the literature, the phytoadaptogens are able to increase performance and reduce fatigue, modulate the inflammatory response in the experiment and in clinical settings.
The aim of the study was to evaluate behavioral disorders in experimental metabolic syndrome and the possibility of treatment with complex phytoadaptogens.
The experiment was carried out on male Wistar rats (9-10 weeks old, weighing 330±20 g, n=30) obtained from the Rappolovo nursery (St. Petersburg, RF). The animals were placed in a room with artificial lighting (12/12) and adjustable temperature (21±1 °C) and humidity (50-55%). The rats were kept in cages (5 animals in each); food and water were provided ad libitum.
The study was approved by the Ethics Committee of the Institute of Biomedical Research – a Branch of the Vladikavkaz Scientific Center of the Russian Academy of Sciences (Minutes No. 7 dated February 20, 2019). The study was conducted in accordance with the ethical standards established by the Declaration of Helsinki.
After the first adaptation period (2 weeks), the animals were randomly divided into 3 experimental groups: Group 1 for control, Group 2 for metabolic syndrome, Group 3 for the treatment of metabolic syndrome with complex phytoadaptogens. Animals in Groups 2, 3 were fed a high-carbohydrate, high-fat (HCHF) diet.
The HCHF diet contained 175 g of fructose, 395 g of sweetened condensed milk, 200 g of beef tallow, 155 g of powdered rat food, 25 g of Hubbell, Mendel and Wakeman salt mixture, and 50 g of water per kilogram of food. In addition, drinking water for the MS group was supplemented with 25% fructose. The total feeding time was 16 weeks. The presence of metabolic syndrome in the animals was confirmed by biochemical, pathomorphological and functional study methods according to the experimental model used [6]. Group 2 rats were euthanized after 16 weeks of feeding to assess the progression of the pathophysiological changes of metabolic syndrome.
After 16 weeks of the diet, Group 3 rats received complex phytoadaptogens for 14 days. The CPhA extract consists of standard 70% extract of Glycyrrhiza glabra and 40% extract of Rhodiola rosea, Acanthopanax senticosus in a ratio of 1:2:1 [7]. The dose was calculated based on the average daily volume of consumed fluid and the coefficient (x10) for small laboratory animals (0.1 mL/100 g) per day.
Behavior was recorded and calculated using computer software for animal activity monitoring (RealTimer, OpenScience, Russia) in the open field and elevated plus maze tests.
The open field test is a square arena with sides equal to 100 cm and a height of 40 cm, divided into equal 25 squares (40×40×30 cm3). It has been shown that testing in an open field with a gray arena does not reveal intergroup differences in behavioral parameters in animals. Due to the transition between habitats, a special type of stress behavior occurs in the arena of a gray open field, with manifestations suggesting a mixed state of anxiety and phobia regardless of the animals’ predicted stress tolerance. We therefore did not divide animals in this experiment into groups according to their resistance to stress [8]. Parameters assessed in the open field test included horizontal activity (distance expressed in squares), vertical activity (number of rearings), number of grooming and defecation events.
The elevated plus maze is a plus-shaped apparatus with four arms connected at right angles to each other, as described by Handley and Mithani. The elevated plus maze consists of two closed (30×5×30 cm3) and two open (30×5×1 cm3) arms perpendicular to each other and connected by a central arena (5×5 cm2). The closed arms have a high wall (16 cm), while the open arms have no side wall. Rats were placed in the central platform and faced a closed arm. Evaluated parameters included total time spent in open and closed arms. An arm entry was recorded once a rat had all its four limbs inside the arm.
On the day of the test, the rats were transported to the test room for 2 hours. Each rat was then placed in the same corner of the open field and elevated plus maze arena, and behavior was recorded for 5 minutes in both tests. To avoid the presence of olfactory cues, all equipment (open field, elevated plus maze) was thoroughly cleaned with 20% ethanol and then wiped with dry paper after each test. The tests were performed between 09:00 a.m. and 02:00 p.m.
Data analysis was carried out using GrafPadPrism 8.03 software (USA). The distribution of continuous variables was tested for normality using the Shapiro-Wilk test. The Kruskal-Wallis test was used to compare independent datasets. The Wilcoxon test was used to compare dependent datasets. Median (25–75‰) values were provided as descriptive statistics due to the small number of values in the sample. p-values <0.05 were considered statistically significant.
Behavior is one of the ways for animals to actively adapt to environmental influences. An animal experiences stress when placed in the experimental “open field” setup, which is shown by its behavior in the first 4 minutes of the test [3].
Rats with metabolic syndrome (MS) (Group 2) showed a trend towards a decrease in horizontal locomotor activity (HLA) compared to control (Group 1) within 5 min. The number of rearings with a support (vertical locomotor activity (VLA)) on the arena wall in Group 2 showed significant differences with the control (p=0.017) (Table 1). This means that metabolic syndrome is associated with a decrease in orientation-exploratory activity (↓HLA and VLA). In the complex phytoadaptogens group, no significant differences with the control were observed in HLA or VLA, with a recovery of test results within the confidence interval of the control, that is, there was a tendency to normalized orientation-exploratory activity.
The main significant manifestation of strong fear in animals with metabolic syndrome in the OFT was the duration of the immobility period, which was significantly different from control values (p=0.011). This fact confirms the significant differences in this parameter with the control animals in Group 3 (p=0.025). But the use of CPhA resulted in a significant difference with the MS group in terms of periods of immobility (p=0.014).
Table 1. Changes over time in open field test and elevated plus maze parameters in all experimental groups
| Parameters | Control (Group 1) | MS (Group 2) | MS+CPhA (Group 3) |
| Open field test | |||
| Horizontal activity (distance, expressed in squares) | 58.5(36;65.5) | 29.5(25;45) р*=0.017 | 45(33.5;53) |
| Vertical activity (number of rearings with support on the wall) | 9(5.5;13) | 2.5(2;6) р*=0.017 | 7(3.5;8.5) |
| Vertical activity (number of rearings without support on the wall) | 7(3;8.5) | 6.5(4;9) | 5(3;7) |
| Period of immobility (sec) | 20(15;32.5) | 60(45;75) р*=0.011 | 39(32.5;45) Р*=0.025 P**=0.014 |
| Number of grooming events | 7(3.5;13.5) | 27.5(20;40) р*=0.011 | 15.5(14.5;16) Р**=0.017 |
| Elevated plus maze | |||
| Number of entries | 3(0;5) | 0(0;2) р*=0.043 | 0(0;0,5) |
| Number of defecation events | 3(2;3) | 4,5(2;6) р*=0.017 | 0(0;2) |
| Number of grooming events | 2(2;3) | 2(1;3) | 2(1;3) |
Note: Control (healthy animals), MS (metabolic syndrome), MS + CPhA (treatment of metabolic syndrome with complex phytoadaptogens), the results are presented as Me (25‰;75‰); significant differences: *- vs. control, ** - vs. MS; P<0.05.
The grooming response in laboratory animals in a situation of emotional stress is a mixed response as a consequence of emotional stress following the impact of frightening stimuli; it is closely related to the immobility time. In particular, the duration of grooming was significantly different from the control in Group 2 (p=0.011). The CPhA group showed significant differences with MS in grooming activity (p=0.017).
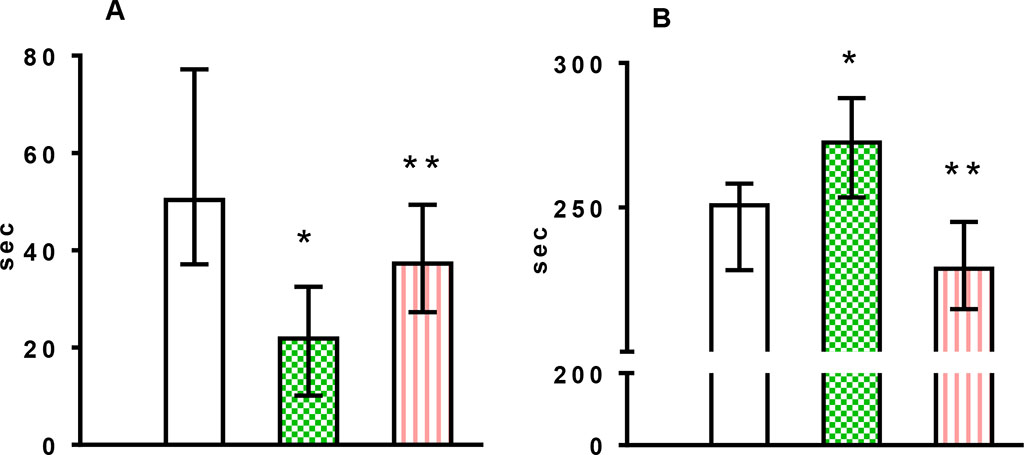
Figure 1. Changes over time in elevated plus maze parameters in all experimental groups (A, light arm time; В, dark arm time)
Note: Control (healthy animals), MS (metabolic syndrome), MS + CPhA (treatment of metabolic syndrome with complex phytoadaptogens), the results are presented as Me (25‰;75‰); significant differences: *- vs. control, ** - vs. MS, p<0.05.
Analysis of the parameters of the elevated plus maze test revealed increased anxiety and symptoms of fear in animals. In Group 2 rats (MS), the following differences with the control were observed: decreased time spent in the open arm (p=0.012) (Figure 1A), increased time spent in the dark arm (p=0.043) (Figure 1B), increased number of entries (p=0.043) (Table 1), which indicated increased anxiety. In the complex phytoadaptogens group, the time spent in the open (Figure 1A) and closed (Figure 1B) arms of the maze did not significantly differ from the respective values in the control (Figure 1).
The number of defecation events was increased in the metabolic syndrome group compared with the control (p=0.017), reflecting the emotional responses of the animals. In the treatment group, the obtained result did not significantly differ from the control (Table 1).
Almost all overweight patients have an eating disorder. The development of eating disorders is associated with specific personality traits in obese patients, such as decreased stress resistance, a tendency to anxiety and depressive responses, increased emotionality [4]. Understanding the links and interactions between stress, neurobiological adaptation, and obesity is important for developing effective prevention and treatment strategies.
The experiment showed that metabolic syndrome is associated with increased anxiety and fear. Data obtained during simultaneous OF and EPM tests indicate an anxiolytic efficacy of the complex phytoadaptogens: a tendency to normalization of open field test (horizontal and vertical locomotor activity and periods of immobility) and elevated plus maze (time spent in open and closed arms, number of entries, number of defecation events) results within the confidence interval of the control. This indicates a decrease in the manifestations of fear and anxiety, which was evident in increased orientation and exploratory activities.
The efficacy of the complex phytoadaptogens can be explained by several mechanisms, most importantly improved adaptation to stress. The autonomic nervous system produces various types of response to emotional stress: changes in body temperature, sweating, cardiovascular and gastrointestinal parameters, respiratory rate. Chronic stress is associated with psychovegetative disorders. Normally, stress leads to the release of corticotropin-releasing factor and, ultimately, glucocorticoids [5]. Glucocorticoids inhibit the secretion of corticotropin-releasing factor by a feedback mechanism, then the system returns to its original state. Corticotropin-releasing factor decreases the synthesis of key neuropeptides, such as brain-derived neurotrophic factor (BDNF). Chronic stress disrupts the feedback mechanism, and glucocorticoids circulate for a long time, which leads to severe neuronal insufficiency in brain structures containing glucocorticoid receptors, for example, the hippocampus. Damage to the hippocampus can lead to a deterioration in the body’s adaptive capabilities during subsequent stressful influences. This can contribute to the development of anxiety, depressive, and somatic symptom disorders [5, 6].
Complex phytoadaptogens can influence the behavior of animals through several mechanisms. They modulate the synthesis of cortisol and adrenocorticotropic hormone during stress, increase the levels of neurohormones (“happy hormones”: endorphins, dopamine), exhibit neuroprotective activity, prolong the resistance stage of the Selye triad [7, 8]; secondary metabolites of CPhA promote cell adaptation to stress, which is called the phenomenon of hormesis or preconditioning [9].
Another possible mechanism is reduction by CPhA of the production of inflammatory mediators in metabolic syndrome. TNF-α is regarded as a mediator of insulin resistance and a regulator of the body’s energy metabolism. TNF-α has been shown to affect the insulin receptor and glucose transporters, potentiating insulin resistance and stimulating leptin secretion [10], and increase the production of IL-6, which also inhibits the metabolic effects of insulin by blocking insulin-dependent activation of signal transducers, insulin-induced synthesis of glycogen. According to the cytokine hypothesis, psychosomatic changes associated with an increase in cytokines can lead to the induction of indoleamine-2,3-dioxygenase (IDO) with the formation of tryptophan (TRP) catabolites, with a subsequent decrease in the availability of TRP and serotonin (5-HT), which also increases anxiety and leads to depression [11].
TNF-α has a direct effect on the development of insulin resistance at the hepatocyte level. Progressive insulin resistance increases leptin resistance. Leptin (“the voice of adipose tissue”) regulates eating behavior by acting on the hypothalamic satiety center; increases the tone of the sympathetic nervous system; enhances thermogenesis in adipocytes; inhibits the synthesis of insulin; acts on the insulin receptor of the cell, decreasing the transport of glucose. In MS, however, leptin does not fulfill its basic biological functions. The increased anxiety of animals with MS can be explained by the development of leptin resistance in response to a diet rich in carbohydrates and fats, in particular, resistance to leptin in its main targets, the hypothalamus [12] and the ventral tegmental area [13]. This condition possibly develops due to a decrease in leptin receptor gene expression accompanied by a decrease in signaling and/or a putative decrease in leptin transport into the cerebrospinal fluid in insulin resistance [14, 15]. It is generally accepted that the amygdala plays a major role in the modulation of anxiety, and dopaminergic receptor mechanisms play an important role in this modulation. Since neurons in the ventral tegmental area are the source of dopamine innervation of the amygdala, leptin resistance in this area may underlie the anxiety behavior observed in our experiment.
Another peripheral hormone that plays an important role in the regulation of eating behavior is ghrelin. As discussed above for leptin, ghrelin has recently been implicated in stress-induced eating and behavioral changes. It has been shown that activation of ghrelin signaling pathways in response to chronic stress may be a homeostatic adaptation that helps humans cope with stress, but at the cost of increased caloric intake. Catecholamines secreted in response to stress appear to directly stimulate ghrelin cells. Like leptin, ghrelin is a potent modulator of mesolimbic dopaminergic circuits [16].
The third aspect is that chronic low-intensity inflammation leads to increased oxidative and nitrosative damage to neurons, pancreatic cells, and endothelium. The oxidative and nitrosative stress (O&NS) pathways are linked in a vicious circle in which immune-inflammatory responses deplete endogenous antioxidants and reactive oxygen species (ROS) activate pro-inflammatory promoter genes through intracellular signaling cascades, such as mitogen-activated protein kinases (MAPKs) and NF-kB; in addition, an alteration of insulin-producing cells in the pancreas and an increase in IR are observed [16].
Thus, we see a number of overlapping pathophysiological mechanisms that contribute to the development of behavioral disorders in chronic low-intensity inflammation against the background of MS, which determines the observed positive effects of the complex phytoadaptogens exerted at the central (CNS), systemic (hormonal regulation) and cell levels.
The complex phytoadaptogens inhibit the transcription factors NF-kB (receptor activator of nuclear factor kappa-B (RANK)) and FOXO3a (a key transcription factor that regulates cell response caused by oxidative stress), resulting in neurons adapting to the stress. Glycyrrhizin from Glycyrrhiza glabra significantly suppresses the expression of nuclear factor of activated T cells, cytoplasmic 1 (NFATc1); reduces the secretion of tumor necrosis factor-α (TNF-α), IL-1β and IL-6, decreases the production of reactive oxygen species, inducing the phosphorylation of AMPK (AMP-activated protein kinase), which leads to an increase in the activity of antioxidant enzymes [17-19].
Acantopanax senticosus extract increases the activity of antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, catalase, in the liver of experimental mice with obesity / type 2 diabetes, reduces the accumulation of ROS (superoxide anion radical and Н2О2) [9]. The best-known plant-derived antioxidant is Rhodiola rosea, which enhances the endogenous antioxidant enzyme response. The use of Rhodiola rosea extract inhibits the activity of proline dehydrogenase (PDH) and glucose-6-phosphate dehydrogenase (G6PDH). Inhibition of PDH and G6PDH activity by Rhodiola rosea prevents the oxidation of proline required for the production of ATP, which is associated with an endogenous antioxidant enzyme response through the proline-mediated pentose phosphate pathway, which consequently leads to inhibition of adipogenesis. Rhodiola rosea extract and its main biologically active substance, tyrosol, increase superoxide dismutase activity, which leads to a decrease in the content of reactive oxygen species during adipogenesis [20]. When used in a complex, the phytoadaptogens modulate and potentiate each other’s effects, which ensures their protective effect.
In conclusion, the results support the idea that a high-carbohydrate, high-fat diet induces metabolic syndrome in rats, which contributes to the behavior disorder including impaired exploratory activity and increased anxiety. However, further research is needed to unravel the mechanisms underlying the behavioral changes in leptin resistance that may underlie the anxiety behavior observed in our experiment. The biologically active substances of complex phytoadaptogens can be a new, promising component in the treatment of anxiety and depressive disorders in metabolic syndrome, acting both through the autonomic nervous system and central structures which are targets for leptin and ghrelin, and as protectors of neurons and peripheral tissues from oxidative and nitrosative stress, eliminating insulin resistance and decreasing the release of cytokines.