- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.4
Sepsis is a serious disease accompanied by the development of a systemic inflammatory response syndrome. The informativeness of existing diagnostic markers of sepsis at various stages of its development remains controversial. Research into septic conditions has led to significant progress in determining the body’s response to infection. The search for laboratory indicators capable of indicating the prognosis of the disease is an important diagnostic criterion in the timely treatment and prevention of sepsis in hospital treatment. The publication presents data from a survey of the studied changes in arterial blood parameters in septic patients with a prognostic assessment. The indicators of biomarkers of inflammation in septic patients were analyzed, the tinctorial and other bacteriological properties of pathogens in the development of generalized sepsis were evaluated, the components influencing the favorable and unfavorable outcome of the disease were determined in the research. The identification of laboratory parameters that help to give the real assessment of the severity and prognosis of the disease in patients of the intensive care unit in a timely manner will allow you to choose effective treatment methods, timely adjust the prescribed therapy and reduce the number of deaths.
Keywords: biomarkers of inflammation, bacteriological examination, sepsis, intensive care unit (ICU) patients, procalcitotnin (PCT), blood gases, systemic inflammatory response syndrome.
Sepsis is a serious disease accompanied by the development of systemic inflammatory response syndrome (SIRS) caused by infections which leads to the death of every fourth patient. Millions of people around the world suffer from organ dysfunction syndrome, which occurs as a result of an inappropriate reaction of the body to infection [1].
Sepsis is diagnosed in 1-2% of all hospitalized patients and in 25% of patients in intensive care units (ICU). The percentage of deaths in this pathology is 20%, in severe sepsis – 40%, in septic shock – more than 60% [2].
Since there are no specific clinical symptoms and signs in sepsis, its diagnosis is difficult. This leads to a constantly emerging need for early diagnosis of sepsis and its appropriate treatment [3].
The effectiveness of therapeutic measures largely depends on the early diagnosis of septic condition by monitoring the symptoms of multiple organ dysfunction in severe patients with a clear focus of infection, as well as the search for infection in patients with multiple organ failure. The information about early factors that allow predicting the course of the disease and its lethality is limited. Early diagnosis and the initiation of appropriate treatment in the first hours after confirmation of the diagnosis improve the prognosis. To solve this problem in clinical practice, the level of biomarkers of blood inflammation is determined, which before the pathogen is detected it may indicate the presence of infection, its nature and may also determine the severity of sepsis and the effectiveness of therapy [4,5].
The identification of laboratory parameters that help to give an adequate assessment of the severity and prognosis of the disease in ICU patients in a timely manner will allow selecting effective treatment methods, timely correcting the prescribed therapy and reducing the number of deaths. Thus, the search for laboratory indicators capable of indicating the prognosis of the disease is relevant.
The aim of the study was to study the prognostic assessment of laboratory parameters in septic patients in the intensive care unit and intensive care unit.
The object of the study: peripheral and arterial blood of septic patients in the intensive care unit.
The subject of the study was a comparison of blood parameters in patients with favorable and unfavorable outcomes with a confirmed diagnosis of sepsis.
The study was conducted on the basis of a clinical hospital. Two groups of patients were examined who were treated in the intensive care unit, anesthesiology and intensive care unit No. 1 with a confirmed diagnosis of sepsis at the age of 36-60 years, which corresponds to the 2nd maturity period of age periodization. The criterion for selecting patients was the presence of a positive hemoculture and the level of procalcitonin above 0.5 ng/ml. The information about the patient, their clinical diagnosis, discharge and postmortem epicrisis were obtained from the patient's medical history. The following tests were performed in the study: a blood test for PRC (procalcitonin), a blood test for CRP (c-reactive protein), a microbiological blood test for sterility and an analysis of arterial blood gases. The collection of material and research was carried out in the first days of admission to the resuscitation in intensive care units (ICU).
Among patients with septic process 26 (67%) gram-negative pathogens and 13 (33%) gram-positive pathogens were detected. While assessing the tinctorial properties of pathogens, Gram staining revealed 56% gram-positive and 44% gram-negative bacteria in the group with a favorable outcome which significantly differed from the data of the group with a fatal outcome, where 83% gram-negative and 17% gram-positive were detected (χ2=6.412, p=0.012), while four patients had several pathogens were detected simultaneously. Hence it can be concluded that gram-positive pathogens prevailed in patients with a favorable outcome and gram-negative pathogens prevailed in patients with a fatal outcome. Thus, the presence of gram-negative pathogens and the presence of several microorganisms increases the likelihood of death. This may be due to the fact that gram-negative microorganisms in their cell wall carry LPS (lipopolysaccharide), which is a powerful endotoxin of the bacterial cell wall, contributing to damage to the microcirculatory bed and the development of septic shock [6]. The diagnosis of sepsis has a high risk of death, therefore, it is relevant to search for laboratory indicators that will make it possible to make a more accurate prognosis of this disease.
The result of the study revealed that CRP, which is a non-specific biomarker of sepsis inflammation in the groups exceeded the reference values of the norm. In the group with a favorable outcome, it was 150.9 ± 26.90 mg/l, which was slightly lower by 4.9 mg/l than the level of CRP in the second group. The level of CRP in persons with a fatal outcome on average reached 155.20 ± 18.78 mg/l. There were no statistical differences between the groups for this indicator (p>0.10).
When assessing the level of procalcitonin, which is a specific biomarker of sepsis inflammation, it was found that in the first group the protein level (PRC) was 5.1 ± 1.92 ng/ml, which was statistically significantly lower than the PRC level in the second group of 12.1 ± 1.96 ng/m (p <0.02).
Thus, the study of inflammation biomarkers showed that an increase in the level of CRP and PRC in the study groups. At the same time, statistical differences in the groups were found only for PRC.
According to the literature data an increase in the level of PRC in dynamics may indicate an unfavorable prognosis of the disease [6]. This may be due to the fact that the level of PRC 2-10 ng/ml indicates sepsis with a high risk of developing multiple organ failure and an increase in the level of PRC over 10 ng/ml indicates organ dysfunction resulting from severe bacterial sepsis or septic shock [7].
Analysis of arterial blood gases in particular partial pressure of oxygen and carbon dioxide did not reveal significant differences between the groups. In the group with a favorable outcome, PaO2 and RaSO2 levels reached 90.5 ± 8.51 mmHg and 40.8 ± 4.27 mmHg, respectively, which was slightly higher than in the second group where PaO2 was 86.1 ± 5.94 mmHg, and PaCO2 decreased to 35.9 ± 3.13 mmHg (Fig. 1.).
Thus, in the first group arterial blood gas values were slightly higher than in the second group. The decrease in PaO2 may be due to the development of hypoxemic respiratory failure resulting from microcirculatory disorders in the pathogenesis of sepsis. A decrease in RaSO2 is possible with hyperventilation of the lungs against the background of respiratory alkalosis, which is a compensatory mechanism of lactic acidemia [8].
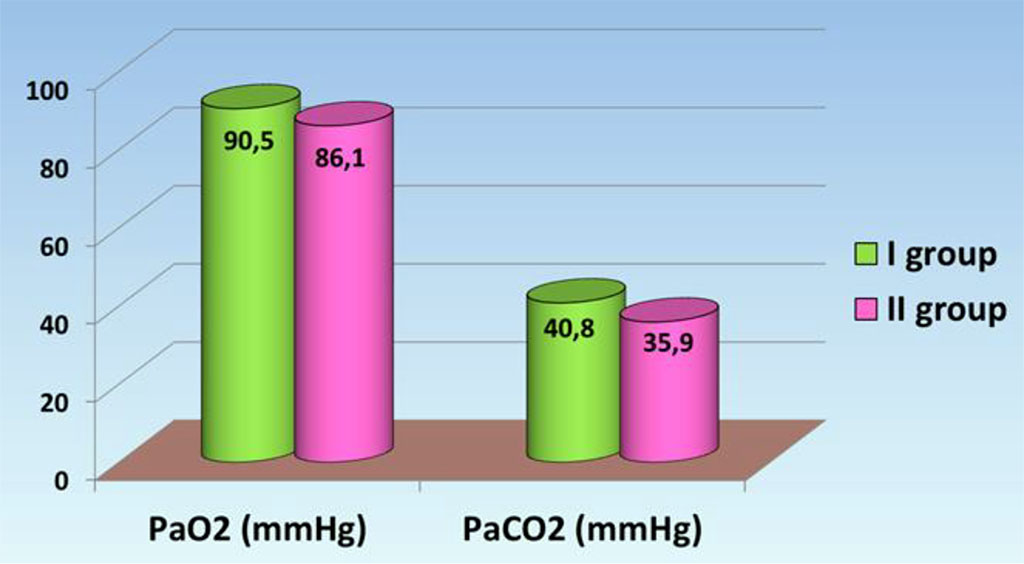
Fig. 1. Parameters of arterial blood gases in septic patients.
The indicators of the acid-base state of arterial blood were evaluated.
In the group with a favorable outcome the average pH values (7.40 ± 0.01), the indicators of the standard excess of bases (-1.5 ± 1.02) and standard bicarbonate (23.3 ± 0.84) were slightly higher than the same values in the second group (7,34 ± 0,04, 4,27 ± 2,78, 21,6 ± 0,94) accordingly at the same time statistically reliable there were no differences in the groups.
Based on the parameters of pH, PaCO2, Base and HCO3 the type of acid-base equilibrium displacement was calculated. Thus, in the first group 12% of patients with acidosis, 38% with alkalosis and 6% with a mixed form were identified and in the second group 44% of patients with acidosis and alkalosis and 7% with mixed forms were found.
Thus, it was revealed that patients had multidirectional changes in the (ABS acid-base state) both in the acidic and in the main direction. Thus, in the group with a favorable prognosis the majority of patients (44%) did not experience a shift in acid-base equilibrium, in the group with an unfavorable prognosis acidosis and alkalosis prevailed equally Based on the analyzed sources, it was found that in septic patients alkalosis is in the first place and acidosis is in the second place, while the highest mortality was observed from acidosis [9-11].
Oxmetric indicators are characterized by the level of hemoglobin and saturation. In the group with a favorable outcome the level of hemoglobin and saturation was 102.4 ± 2.17 g/l and 93.2 ± 1.77%, respectively, which was slightly lower than in the group with a fatal outcome and amounted to 104.4 ± 7.15 g/l and 94.4 ± 1.39%, respectively. At the same time there were no significant differences in oximetric indicators in the groups. But in both groups there was a decrease in hemoglobin and saturation parameters which may indicate tissue hypoperfusion and hemic hypoxia [12].
In the first group the levels of potassium (4.1 ± 0.14 mmol/L), sodium (140.4 ± 0.58 mmol/L) and chlorine (104.1 ± 1.01 mmol/L) were slightly lower than the levels of potassium (4.3 ± 0.29 mmol/L), sodium (143.7 ± 1.68 mmol/L) and chlorine (107.5 ± 1.65 mmol/l) of the second group, while the average values in the groups are within the reference limits.
The level of ionized calcium was reduced in both groups (0.9 ± 0.06 mmol/l and 0.8 ± 0.05 mmol/L), respectively. There were no significant differences in electrolytes.
The osmolality level in group I was 289.4 ± 2.09 mosm/kg which was statistically significantly (p<0.02) lower than the osmolality level in group II of 298.8 ± 3.0 mosm/kg. At the same time the average values in the groups are within the reference limits (Pic. 2).
Thus, the study of electrolytes showed a decrease in the level of potassium, sodium and chlorine and osmolality in the first group compared to the second. There was also a decrease in the concentration of ionized calcium in both groups. At the same time, statistically significant differences in the groups were revealed only for osmolality.
Hyperchloremia is closely related to metabolic acidosis, since an increase in the concentration of chlorine leads to a decrease in the ionic difference which increases H+, leading to acidosis. The severe course of hyperchloremic acidosis is associated with its effect on hemodynamics [8,12].
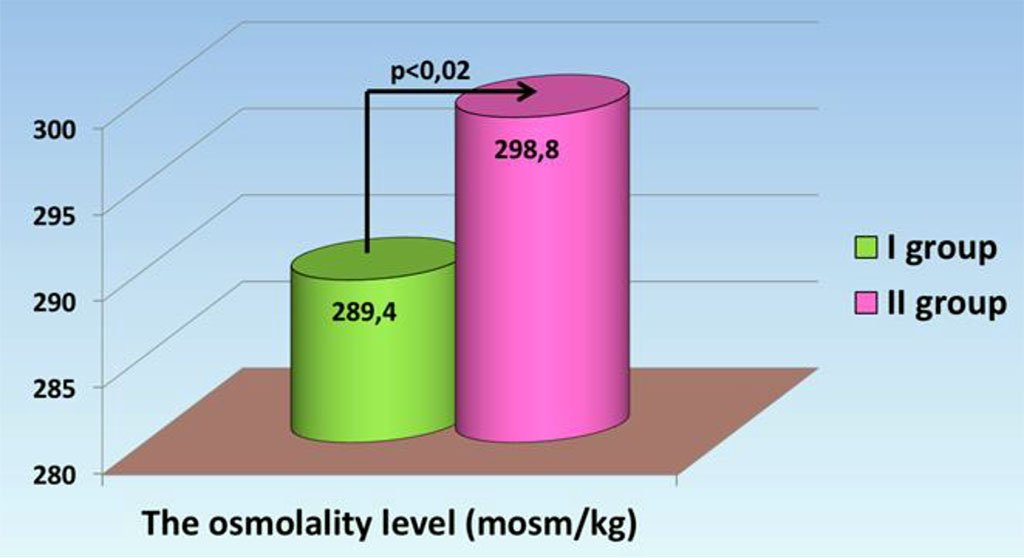
Fig. 2. Osmolality indicators in septic patients.
The following metabolites were evaluated in the study: glucose, lactate and bilirubin.
The glucose level in the first group was 6.8 ± 0.39 mmol/l which was statistically less than the glucose level in the second group (11.4 ± 1.28 mmol/L), while the values in the groups exceed the reference values (p <0.002) hyperlactatemia was detected in the arterial blood of patients of both groups. In the group with a favorable outcome the average lactate level was 2.2 ± 0.42 mmol/l. In the group with an unfavorable outcome the lactate concentration reached 6.4 ± 1.12 mmol/l, which was significantly higher than in the first group (p <0.002).
The lactate and glucose indicators obtained during the study revealed a pattern that can be reflected by calculating the ratio of these indicators. We believe that the obtained coefficient can be used to assess the outcome of the disease. It is known that the values of lactate and glucose in the blood make it possible to predict the possibilities of functioning of the body in conditions of lack of oxygen, which is carried out due to anaerobic glycolysis and gluconeogenesis in the liver and kidneys (Cory cycle). During the study it was found that an increase in lactate indicators and a simultaneous decrease in glucose indicators is characteristic of patients in critical condition, so in the first group their calculated ratio was 0.3 ± 0.04, in the second group the indicator was twice as high and reached 0.6 ± 0.09, which was significantly lower (p <0.02).
The content of total bilirubin was statistically significantly different in the groups (p <0.05), while the average level of total bilirubin in the first group was within the reference values of 12.5 ± 3.85 mmol/l and was lower than the level of total bilirubin in the second group of 49.3 ± 77.00 mmol/l.
Thus, the patients in the first group had an increase in the average glucose and lactate levels, while in the second group the level of all the studied metabolites was increased (Table 1).
Table 1. Prognostic evaluation of arterial blood metabolites
| Indicators | The first group of patients with favorable outcomes, X ± m | The second group of patients with unfavorable outcomes, X ± m | P |
| Glucose | 6,8 ± 0 ,39 | 11,4 ± 1,28 | <0,002 |
| Lactate | 2,2 ± 0,42 | 6,4 ± 1,12 | <0,002 |
| Total bilirubin | 12,5 ± 3,85 | 49,6 ± 17,00 | <0,05 |
Note: P - is the confidence level of the differences.
Thus, a comprehensive assessment of the prognostic significance of blood hemoculture, inflammation biomarkers, metabolites, acid-base and gas composition of arterial blood of septic patients demonstrates multidirectional dynamics in patients with favorable and unfavorable outcomes.
The association of high levels of procalcitonin and lactate with an unfavorable outcome of the septic process was noted.
A favorable prognosis is shown for patients with sepsis in the presence of a single gram-positive pathogen in the blood hemoculture.
For the first time the association of high values of arterial blood osmolality with an unfavorable outcome in the septic process has been shown.
The significance of the study lies in the fact that for the first time a coefficient was proposed reflecting the dynamics of lactate and glucose in arterial blood and increasing in patients with high risks of an unfavorable outcome in the septic process.