- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.3
A novel coronavirus infection was described in 2019 in Wuhan, China. From the first months of the spread of the infection around the world, evidence began to appear that patients after recovery had various symptoms. Duration, intensity, and variability of symptoms vary among patients and are often not associated with the severity of the most acute illness. Recently the concept of post-COVID syndrome (post-COVID or long-COVID in the English-language literature) has acquired increasingly clear diagnostic criteria. Persistent symptoms and / or the appearance of delayed complications after 4 weeks or more from the onset of symptoms of an acute illness are commonly called post-COVID syndrome.
The wide range of symptoms that can occur in patients with post-COVID syndrome is now a major health concern worldwide. A proper clinical evaluation will help determine the etiology and build a treatment plan. Longer studies aimed at identifying the effects of COVID-19, possible risk factors for their development, a detailed study of the pathogenetic mechanisms of SARS-CoV-2, and the development of treatment and rehabilitation methods to improve the mental and physical health of surviving patients are relevant elements of study for the foreseeable future.
T-lymphocytes are a poorly studied population of T lymphocytes. These cells are more often localized in the mucous membranes of the body which have the properties of innate and acquired immunity. The main biological functions are cytolysis, immunoregulation which indicates an important immunocompetent role of this type of cell population in severe infectious diseases. This article provides information on the fraction of T-lymphocytes during the formation of adaptive immunity in patients with post-COVID syndrome.
Keywords: immune status, T-lymphocytes, cellular immunity, immunoglobulins, leukocyte formula, differentiation clusters, gamma-delta t-lymphocytes, flow cytometry, long-COVID
Soon after the start of close scientific observation of the development of COVID-19 disease it became clear that in addition to differences in severity among patients with acute forms of SARS-CoV-2 infection asymptomatic carriers of the disease are possible, as well as other outcomes that manifest themselves in the long term to which primarily includes post-covid syndrome [1,5,7,9,11].
Post-COVID-19 syndrome is a consequence of a coronavirus infection in which up to 20% of people who have had a coronavirus infection suffer from long-term symptoms lasting up to 12 weeks and in 2.3% of cases longer. The post-COVID syndrome is included in the International Classification of Diseases heading code U09.9 “Post-COVID-19 condition unspecified” which also includes the post-COVID state [2].
A number of researchers emphasize that post-COVID syndrome is a fairly broad diagnosis with psychophysiological consequences. The severity of the transferred coronavirus infection directly affects the severity of symptoms after the disease. This is due to the fact that the virus remains in the body and is detected in immunosuppressed patients and even in people with a normal immune system who do not have symptoms of the disease, the virus stays in the small intestine and nervous system, in monocytes isolated from the peripheral blood of patients and in macrophages. And there is also evidence of the ability of the virus to multiply in certain types (CD4+) of lymphocytes [3,4,8,10].
Gamma/delta T-lymphocytes are a poorly understood heterogeneous population of T-lymphocytes that dominate in the mucous membranes and combine the properties of both cells of innate and acquired immunity. Circulating and resident gamma/delta T lymphocytes are isolated. Circulating gamma/delta T-lymphocytes average 1-10% of peripheral blood mononuclear cells [6]. Gamma delta T cells are an important subset of "non-traditional" T lymphocytes because they have the ability to recognize a wide range of antigens without the presence of the major histocompatibility complex (MHC) molecules. They can attack target cells directly through their cytotoxic activity or indirectly through the activation of other immune cells [12]. CD16 is expressed by the majority of gamma delta T cells in the peripheral blood and by some clones of gamma delta T cells which provides their cytotoxic activity.
In connection with the above, the purpose of the study was to identify the fraction of T-lymphocytes, in particular gamma-delta T-lymphocytes, during the formation of adaptive immunity in post-COVID syndrome by flow cytometry.
The study involved 23 women, employees of a medical institution aged 36 - 45 years which corresponds to the 2nd period of maturity of age periodization (A.A. Markosyan, 1965). The mean age was 43.3 ± 2.6. According to the analysis of case histories the subjects did not have chronic pathology and acute pathological conditions. The control group consisted of practically healthy people belonging to the first health group who were examined as a result of a medical examination (n=21). All subjects examined were female. The average age was (40.2 ± 4.51).
The immune status was assessed. In the study of cell populations of T-lymphocytes involved in the immune response during a viral infection, the method of typing monoclonal antibodies was used using flow cytometry (flow cytometry) on an Attune NxT flow cytometer from Thermo Fisher Scientific (T-lymphocytes CD3, CD4, CD8, CD16, CD20 , CD25). The sample for the study was the lysed blood of patients who had recovered from coronavirus infection stained with CD4 and CD16 antibodies. The intensity of fluorescence was used to judge the expression of antigens (the number of receptors) on cell membranes. Indicators of humoral immunity (IgG) were studied by enzyme immunoassay on a spectrophotometer. Phagocytosis indices (spontaneous and induced phagocytosis) by microscopy and immunological test with tetrazolium blue.
As a result of the study of white blood cells the indicators were within the reference values, however a decrease in the number of leukocytes in group I and an increase in lymphocytes were revealed in comparison with the control group (group II) (Figures 1,3).
The Ig M in group I were 3,28±0,63 g/l and control group – 2,54 ± 0,17; Ig G- 18,6 ± 0,9 g/l in group I and 14,16 ± 0,49 control group.
Analysis of the leukocyte formula demonstrates the predominance of myelocytes, the absence of metamyelocytes and a sufficient number of stab neutrophils with a low value of granulocytes in general in the experimental group which is presumably associated with immunosuppression which provokes a high production of cellular elements of myelopoiesis and the release of young immature forms into the peripheral blood in patients with postcovid syndrome (Figure 2).
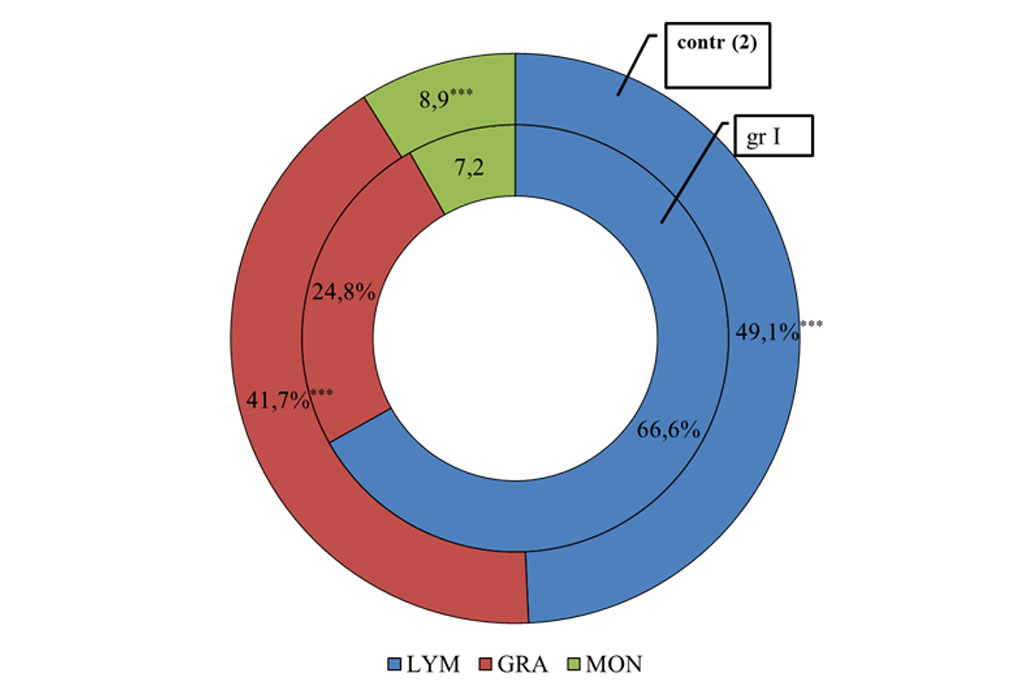
Figure 1. The distribution of different pools of leukocytes in patients with post-COVID syndrome in comparison with the control group.
Note: *** - significance of differences at P<0.001. Distribution of different pools of leukocytes in patients with post-COVID syndrome in comparison with the control group. *** - Significance of differences at P<0.001.
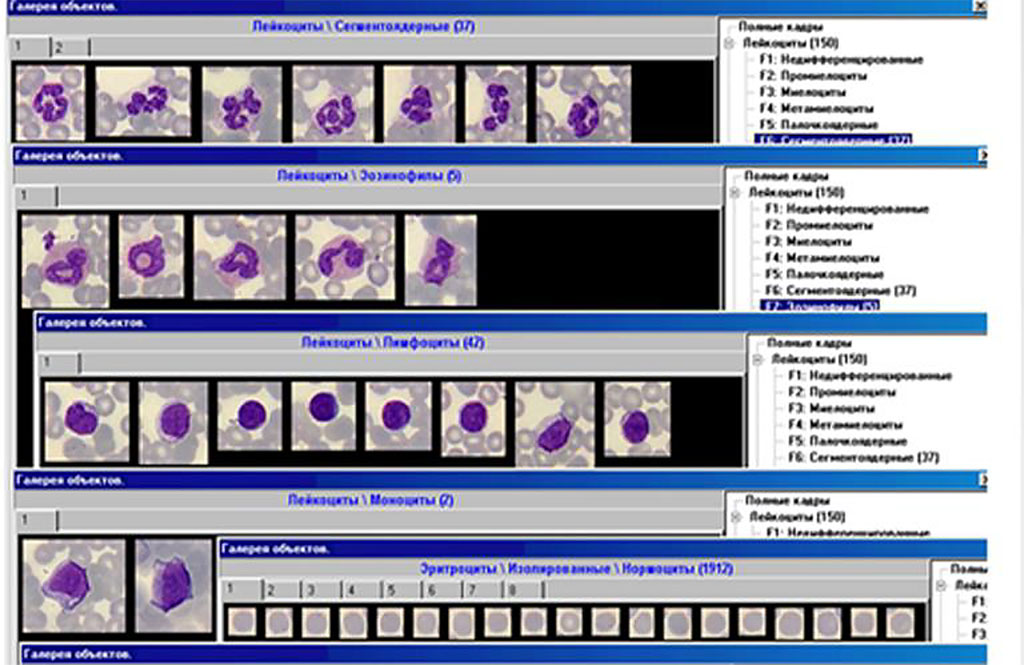
Figure 2. The indicators of leukocyte formula.
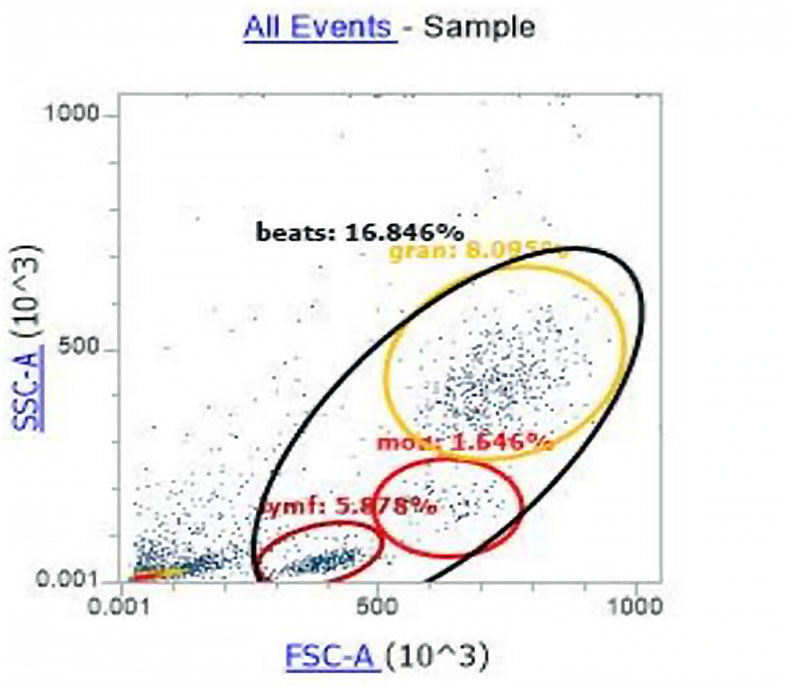
Figure 3. Histogram of the distribution of cell populations according to the principle of direct and side light scattering.
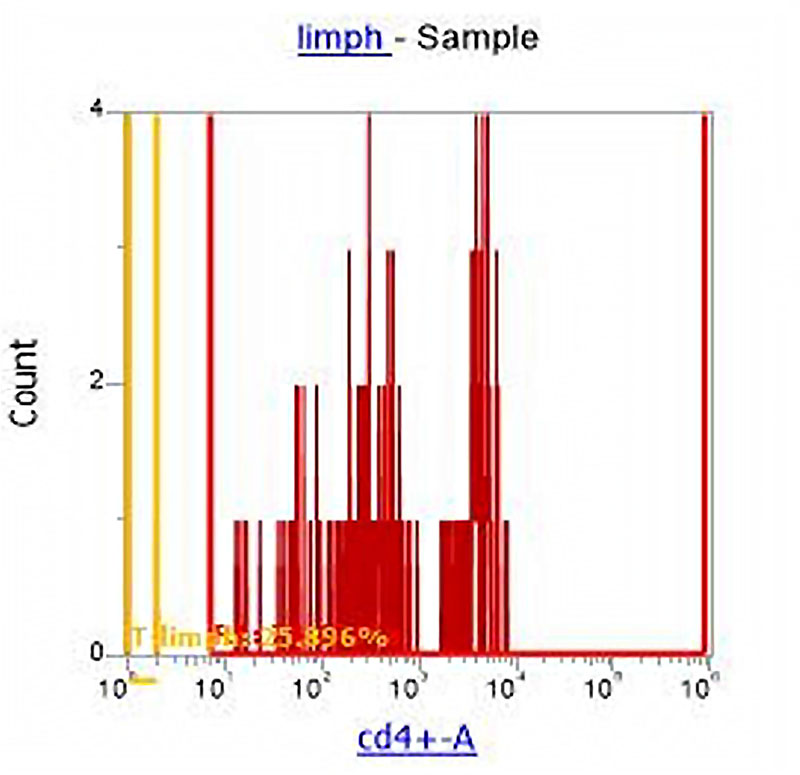
Figure 4. Division of the cell population of lymphocytes into subpopulations: T-Helper (CD4+) and other lymphocytes (CD4-).
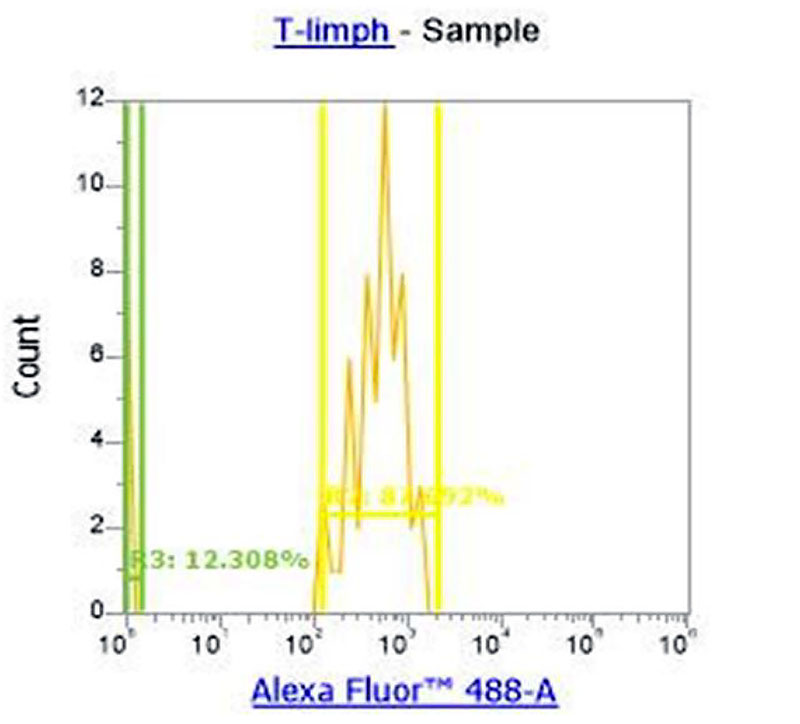
Figure 5. Separation of T-cell lymphocyte subpopulations into CD16+ (gamma/delta T-lymphocytes) and CD16- (other T-helpers).
With the help of CD4 monoclonal antibodies which were previously stained with blood we divided all lymphocytes into CD 4+ (t-helpers) and CD4- (all other lymphocytes) (Figure 4). Data analysis shows a downward trend in the percentage of CD4+ T-helper cells which was only 25.9% in group 1. The percentage of CD 16+ cells was 12.3% with reference values of 5-7% (in some sources up to 10%) which characterizes a noticeable increase in the fraction of gamma / delta T-cells (Picture 5) relative to the total number of T-lymphocyte population in the experimental group.
During the study by flow cytometry using monoclonal antibodies we were able to isolate the fraction of gamma / delta T-lymphocytes among other lymphocytic subpopulations in a group of patients with post-COVID syndrome, data were obtained on the percentage of this type of cells in the peripheral blood of the total number of T-lymphocytes.
Among the fractions of lymphocytes a certain imbalance was observed: with the total number of lymphocytes being within the normal range, there were significantly fewer circulating T-lymphocytes of effector memory (both regulatory and cytotoxic). As a result of comparing the obtained data with the reference values a noticeable increase in the fraction of gamma T-lymphocytes was observed which confirms the data of other researchers on the ability of the SARS-CoV-2 virus to multiply in CD4+ lymphocytes (T-helpers), thereby affecting this population of immunocompetent cells. Therefore an increase in the fraction of gamma / delta T-lymphocytes relative to the total number of T-helpers against the background of a general decrease in the level of T-lymphocytes in post-COVID syndrome suggests that the percentage of gamma-delta T-lymphocytes is increased relative to the total number of T-lymphocytes, since it is gamma/delta T-lymphocytes that are part of innate immunity and form the first wave of adaptive immunity in a non-classical way, thus protecting the body in case of dysfunction of classical adaptive immunity which is an important predictor of the development of an immunodeficiency state after suffering this viral infection.