- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.26
The study, while aiming to study the microarchitectonics and morphology of the mandible alveolar part at dentition defects in 24 males (aged – 50-65; with type 2 diabetes mellitus going through the compensation stage following the correction of bone remodeling imbalance), and 19 healthy persons belonging to the same age group, implied analyzing 3D cross-sections of the adentia zone (through cone-beam computed tomograms), along with bone tissue biopsy histological examination, obtained from the projection area of dental implants. All patients had their biochemical features of bone metabolism within the reference values, the volume of the bone tissue at the missing teeth area was classified as Category A and B (by C.E. Mich, K.B.M. Judy, 1987), while the densitometry value at the lumbar spine and femoral neck (based on T-criterion) fell within the normal value (> -1SD). The X-ray and histological studies carried out in the studied groups, identified no significant differences in the quantitative and qualitative parameters of the mandibular alveolar part within the area of missing teeth. The morphology of the lower jaws alveolar part at the adentia area in healthy patients, which was represented by strongly interconnected trabeculae, single elements of adipose tissue in peritrabecular spaces, small resorption lacunae on the beam surface, few bonding lines, a depleted pool of osteogenic cells observed in the endossal layer – all these point at an age-related decrease in the intensity of reparation processes, as well as at a decreasing rate of bone tissue remodeling and development. The histological specifics of the mandibular alveolar part at the dentition defects in patients with type 2 diabetes, after pharmacological correction of the bone remodeling imbalance by way of interconnected trabeculae, local elements of adipose tissue against red bone marrow, a significant number of activated osteoblasts and inactive flattened osteogenic cell elements, enhanced activity of osteoblastic differon cells, as well as zones of the developing weakly mineralized osteoid, serve proof to an increase in the intensity of bone tissue metabolic processes, the predominance of the bone development phase over resorption and active osteogenesis. The results of X-ray and morphological studies show that in order to bring bone remodeling back to normal and reduce the risk of postoperative complications at the dental implantation in patients with compensated type 2 diabetes prior to dental rehabilitation, a good choice would imply carrying out pathogenetic pharmacological correction using groups of drugs capable of reducing bone resorption and enhancing bone development.
Keywords: diabetes mellitus, dental implantation, bone microarchitectonics, cone-beam computed tomography, histological examination, mandible alveolar part, bone mineral density.
Diabetes mellitus (DM) belongs to the group of socially significant non-communicable diseases, which features epidemic rates of spread increase. As the International Diabetes Federation reports, by 2022 the total number of diabetes cases globally had reached 537 million; the number of undiagnosed cases – 239.7 million; deaths associated with diabetes – 6.7 million, the respective global expenditures having reached $ 966 billion. Besides, there is a growth observed in cases of pre-diabetes, gestational diabetes, as well as an increase in the incidence of type 1 diabetes affecting children [1]. Chronic hyperglycemia, typical of type 2 diabetes, is an aggravating risk factor in terms of triggering severe and progressive lesions affecting the periodontium and the supporting teeth bone, leading to further destruction and loss of the dental attachment. An increase in the number and activity of osteoclasts in case of type 2 diabetes has been proven to lead to a persistent inflammation response in the periodontium, followed by a disturbed integrity of the periodontal ligament and a resorbed alveolar process, as well as a deteriorating development of new bone tissue, all this due to increased apoptosis in osteoblasts and fibroblasts. Periodontal tissue inflammation is to be identified in 100% of patients with type 2 diabetes [2-8].
Patients with type 2 diabetes feature a high and very high degrees of tooth damage caused by carious, as well as a large number of removed teeth, whereas the need for dental prostheses in patients with type 2 diabetes lies somewhere within the range of 78% to 100% [9-14].
A serious complication associated with type 2 diabetes is the musculoskeletal system lesion, the negative factor affecting all bone structures. The pathogenesis of bone tissue damage at consistently high blood glucose values relies on glycation end products (GEP) accumulating inside collagen, which leads to a decrease in the collagen matrix plasticity and a decrease in the bone structure strength. In case of type 2 diabetes, bone renewal and remodeling processes are disrupted, which accounts for the bone tissue not getting updated – due to the GEP, whereas the quality of bone tissue deteriorates [15-17].
The results of bone mineral density (BMD) examination revealed that in case there is no proper control of glycaemia in patients with type 2 diabetes, the BMD shows a more remarkable decrease; improving the glycemic status, though, will quickly lead to a decrease in bone loss. One of the reasons behind the deteriorating bone tissue quality and its increasing fragility is diabetic microvascular complications with impaired microcirculation in the bone tissue [18].
The bone tissue quality is one of the essential factors ensuring the success of implantological treatment, as well it is a reflection of the metabolic processes underway [19-21]. Medicine nowadays views histological and histomorphometric studies as the gold standard for evaluating the bone tissue microarchitectonics, the said types of examination allowing accurate assessment of the bone structural features and qualitative parameters, identifying the scale of the existing changes, and determining the effectiveness of the respective treatment and its effect on the mechanisms remodeling [22-24].
The growing level of diagnostic requirements in dentistry points at the need for non-invasive methods that would help visualize the the perceived bone bed microarchitectonics in the pre-surgery period [25-27]. Computer modeling of jaw bones volumetric reconstructions, taking into account the density of tissues up to the trabecular package, can be done through cone-beam computed tomography (CBCT), which offers such advantages as high resolution; low radiation load; economic feasibility [28-32].
Experts claim that CBCT, employed as a non-invasive method to assess the qualitative parameters of the alveolar bone in order to forecast reparative regeneration and osseointegration during dental implantation, can be used only when the results of a comparative analysis of radiation and histomorphometric studies are available [33-36].
Loss of teeth suffered by patients with type 2 diabetes results in a poorer life quality both due to a deteriorated chewing function and to their aesthetic dissatisfaction, whereas dentition prostheses in such cases will allow not only restoring the dentition functional capacity and aesthetics, yet will also have a positive effect on the course of the underlying disease, improving the glycemia level. Given this, the restoration of the chewing capacity through prosthetic treatment of terminal defects and in case of complete adentia is an important step in the comprehensive course of rehabilitation offered to patients with type 2 diabetes [37-41]. One of the key things in the modern dental rehabilitation when dealing with patients with type 2 diabetes is the expansion of indications for prosthetic treatment of cases featuring partial and complete absence of teeth, while relying on dental implants. There has been some significant progress achieved through the emergence of new drugs for type 2 diabetes, thus allowing patients to remain in the compensation stage for years, as well as progress in the development of new drugs that can have an effect on bone remodeling. The key to successful osseointegration is due to the state of the bone bed, which explains why it is so important to study the microarchitectonics of the bone tissue at the area of planned dental implantation, which offers an objective reflection of the metabolic status [42-45].
Aim: to study the microarchitectonics and the morphology of the mandible alveolar part at the areas featuring missing teeth in patients with compensated type 2 diabetes, and with bone metabolism values falling within the normal range.
The study involved 43 males with partial and complete mandibular adentia, who applied for restoration of dentition defects through dental implantation, and who were divided into 2 groups – the main group and the control group. The main group were patients (n=24) diagnosed with type 2 diabetes mellitus in the compensation stage (E11 – Insulin-independent diabetes mellitus). The control group included healthy patients (n=19) with no general somatic, endocrine pathology and risk factors potentially triggering metabolic osteopathies.
The inclusion criteria for the main group were: sex – male; age – 50 to 65; duration of type 2 diabetes – under 5 years; compensated type 2 diabetes with fasting glucose level <6 mmol/l and glycated hemoglobin (HbA1c) concentration not exceeding 6%; biochemical parameters of bone metabolism – within reference values: total calcium − 2.1-2.6 mmol/l; ionized calcium − 1.06-1.31 mmol/l; parathyroid hormone – 7-53 pcg/ml; calcitonin – 3-13 ng/l; osteocalcin – 3.1-13.7 ng/ml; β-CrossLaps – 0.01-0.6 ng/ml; calcidiol 25(OH)D – 14-60 ng/ml; DEXA results – T-criterion value within normal range > -1SD; lack of general somatic diseases associated with type 2 diabetes; bounded, terminal dentition defects and lower jaw complete adentia (K05.1 – chronic gingivitis, K05.3 – chronic periodontitis, K08.1 – loss of teeth due to accidents, removal, localized periodontitis, ICD-X).
To improve bone remodeling, the main group patients had pathogenetic correction performed prior to dental rehabilitation, whereas the procedure was done involving the following groups of drugs: drugs that reduce bone resorption (calcitonins, bisphosphonates, tetraparatide); drugs of multiple effect (calcium salts, vitamin D); drugs that enhance bone formation (fluorides, anabolic steroids, androgens).
All the patients in the groups in question had the volume of available bone tissue and the degree of the lower jaw alveolar atrophy at the adentia area matching Category A (sufficient volume of bone tissue: width 4 mm or above; height 10 mm or more) and Category B (minimum sufficient volume of bone tissue: width – 2-4 mm; height 10 mm or above), subject to the classification by C.E. Mich, K.B.M. Judy (1987).
To assess the mineral metabolism status in blood serum in the morning on an empty stomach, the following factors were identified: total calcium − by Arsenazo-111 method, on a VitaLab Flexor E (Vital Scientific) automatic biochemical analyzer with an BIOCON 2003 calibrator (Biocon Limited); ionized calcium – employing the ion-selective method on an E-Lyte Plus blood electrolyte analyzer (Medica Corp.); parathyroid hormone – with the LKPP reagent, LPHL, LPHH calibrators, and calcitonin – with LKCL reagent, LCLL, LCLH calibrators by solid-phase chemiluminescent immunoassay on a IMMULITE 2000 XPi automatic analyzer (Siemens Healthcare Diagnostics Inc); osteocalcin by solid-phase chemiluminescent immunoassay on an IMMULITE 2000 automatic analyzer XPi with LKON1 reagent, LONL, LONH calibrators (DPC Cirrus Inc.); β-CrossLaps − by electrochemiluminescent immunoassay on an Elecsys 1010 autoanalyzer, reagent and calibrators 11972308122 (Roche Diagnostics); 25(OH)D − by high-performance liquid chromatography, UV liquid chromatography analyzer, Chromsystems reagents (Chromsystems Instruments & Chemicals GmbH).
The bone mineral density was evaluated with dual-energy X-ray absorptiometry (DEXA) by a General Electric Medical Systems LUNAR PRODIGY Vision 4 machine in the lumbar spine (at the L1-L4 level), femoral neck (Warda triangle), with studying densitograms subject to the T-criterion in SD deviation units. Following the WHO recommendation (1994), the T-criterion, which is the amount of SD from the bone mass peak of persons of the respective sex, shall constitute: norm > -1SD; osteopenia from -1 to -2.5 SD; osteoporosis < -2.5 SD (Fig. 1).
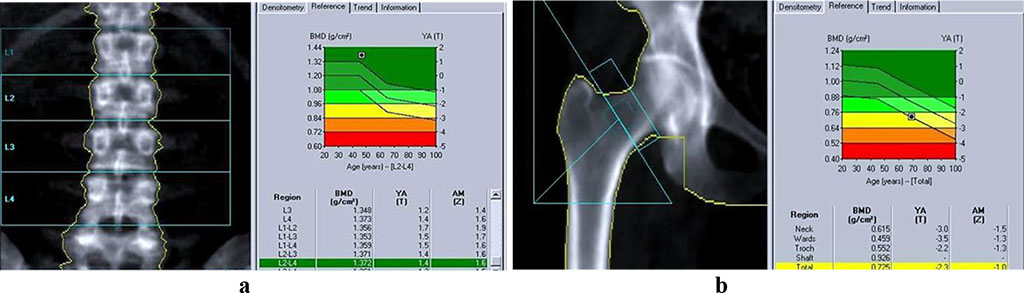
Figure 1. Bone tissue mineral density: a – lumbar spine; b – Warda triangle (GE Lunar Prodigy).
To measure the bone tissue volume in the mandible alveolar part, as well as to study its microarchitectonics, cone-beam computed tomography (CBCT) was performed using a Galileos GAX5 machine (Sirona Dental Systems, Germany). There was also postprocessing of images done involving the construction of panoramic and other multiplanar reconstructions, using the specialized GALILEOS Viewer software. In the basic interface, the studied area was highlighted as the centerline on the panoramic reformat, which was followed with automatic volumetric rendering in a multi-plane format (axial, coronary, sagittal projections, 3D window). The function of selecting the selected layer’s thickness allows segmenting the required area from the entire mass, evaluating the quantitative features of the bone tissue, while in a cross-sectional 3D window it allows detailing microarchitectonics with an evaluation the spongy and cortical bone status (Fig. 2).
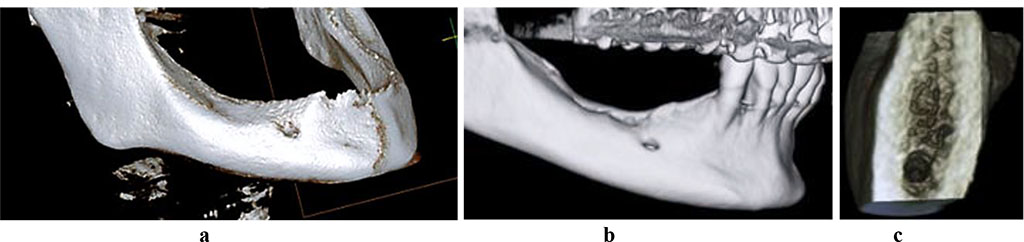
Figure 2. 3D-reconstruction of the mandible with a full (a) and a partial (b) adentia, 3D cross-section of the mandible alveolar part, with neither osteoporosis nor osteopenia observed (c).
To perform histomorphological examination, trepan biopsy was performed through sampling bone tissue while shaping the bed for an implant, using a hollow cutter (Ø=2.0 mm). Following the incision of the mucous membrane and peeling of the muco-periosteal flap, a trepan was used instead of a pilot drill, whereas the former was immersed into the bone to a working depth and removed along with the bone block, thus obtaining a biopsy trepan. The resulting bone material consisted of bone tissue columns with a diameter of 2 mm and a length of 7-8 mm (Fig. 3).
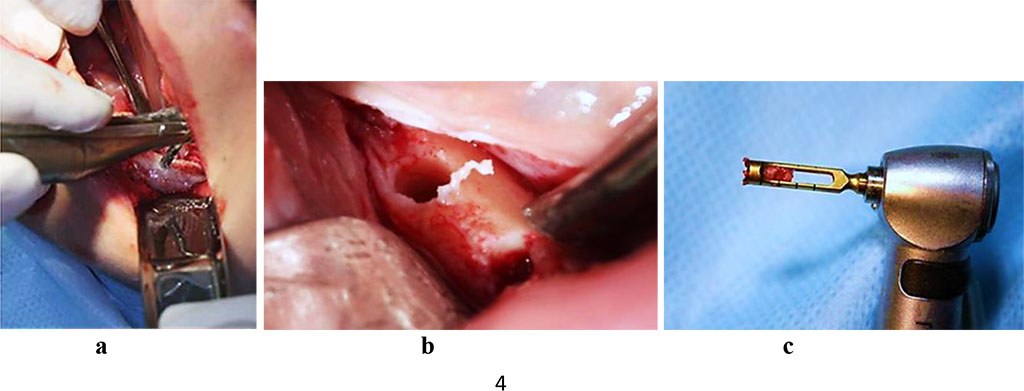
Figure 3. Bone biopsy sampling at the projection area of the dental implant, patient with a partial adentia (a, b), hollow milling cutter with bone biopsy obtained for histomorphological examination (c).
The obtained bone tissue biopsies were fixed in a 10% neutral formalin solution (pH 7.0−7.2) buffered by Lilly, with further biodecalcification (Electrolytic decalcifying solution; Decalcificante elettrolitico, Italy). A histomix paraffin modifier was added to the paraffin thus aiming to improve the quality of the slices (BioVitrum, Russia). Further, following the generally accepted method, the biopsies were poured into molds to obtain paraffin blocks. After pouring into paraffin, sections were made on a rotary microtome (Rotary 3006 EM, PFM Medical), 2.5-3 micron thick. The preparations were stained with hematoxylin and eosin. The histological preparations were studied, analyzed and microphotographed with a ZEISS Axiolab 5 microscope and a ZEISS Axiocam 208 color digital video camera.
The patients of the studied groups had their dentition defects eliminated with Nobel Biocare Parallel CC, Astra Tech, MIS SEVEN, and Dentium Implantium implants, following the standard generally accepted two-stage protocol, the period from installation to implant opening being 3 months (lower jaw). Prior to implantation, all the patients underwent oral sanitation, which implied professional oral hygiene, endodontic treatment and tooth filling, as well as the removal of teeth that could not be treated.
Of the control group 19 patients, 10 (52.6%) featured bounded dentition defects; 6 males (31.6%) had unilateral free-end space defects; 3 (15.8%) had bilateral free-end space defects. The CBCT data showed out of 19 patients in the control group, 17 patients (89.5%) had a sufficient volume of bone tissue (category A based on the classification by C.E. Mich, K.B.M. Judy (1987)) at the area of missing teeth, whereas another 2 patients (10.5%) had a minimum sufficient volume of bone tissue (category B according to C.E. Mich, K.B.M. Judy (1987). The CT scans in the cross-sectional 3D window of the planned dental implantation zone, showed that the compact bone in the lower jaw was uneven in width (thinned at the top of the alveolar ridge, thickened at the periphery). In the mandible dentition defects, the area of the spongy bone was slightly reduced, the bone trabeculae of the spongy bone were wide and attached tightly to one another, intertrabecular space was basically not observable, the vestibular and lingual cortical plates were to be visualized, while the trabecular package was completely intact (Fig. 4).
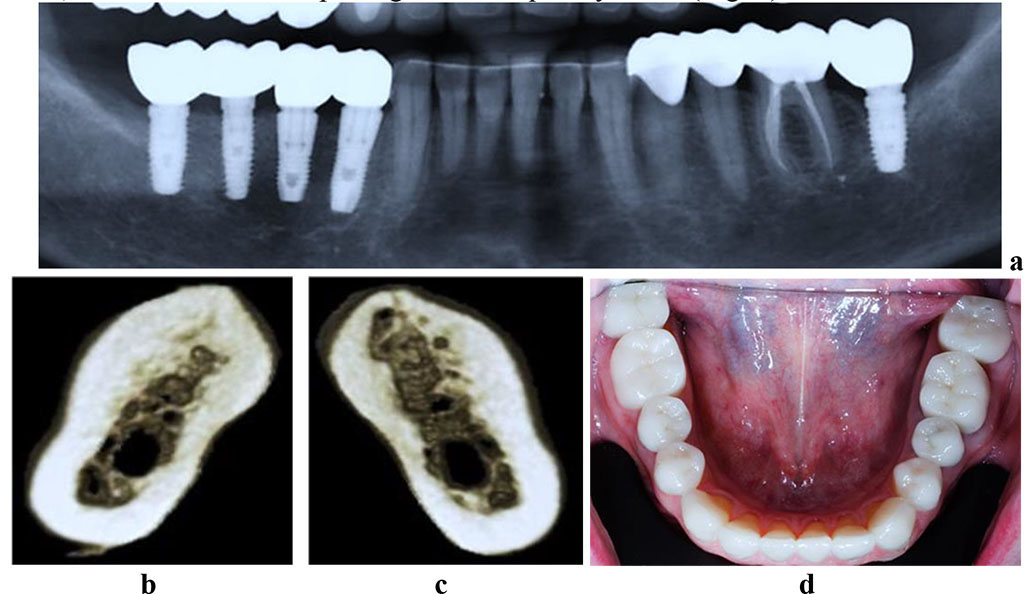
Figure 4. Patient M., 53 y.o. Diagnosis: mandible partial adentia, Class I by Kennedy. Lower jaw CBCT: a – the bone tissue volume 12 months after the dental implant installation (panoramic reformat); 3D cross-sections at the missing teeth area 3.7 (b) and 4.6 (c) prior to the installation of dental implants; d – the lower dentition after implant and prosthetic treatment.
Histological preparations of the mandible alveolar part bone tissue in the control group in the area of missing teeth are represented by the cortical plate and partially – by the spongy substance. The cortical plate has a lamellar structure with Haversian canals of various size. The outer surface contains no periosteum, the surface layers contain immature bone substance. The Haversian canals of a multidirectional course contain a bone marrow reticular stroma with mature vessels. The canals are polygonal. The areas of the intracanal surface feature signs of physiological resorption. There is a small number of osteoclasts and a low osteoblastic activity. The bonding lines are regular, yet rare. The lacunae contain osteocytes. The inner surface of the cortical plate features a smooth contour. The cortical plate and the spongy substance have clearly cut boundaries.
The trabeculae, which belong to a spongy substance, are thickened, the Haversian canals being of varying size. The surface of the trabeculae showed no signs of osteoblastic activity, nor anything like that was to be observed on the inner lining of the Haversian canals. The osteoclast activity in the spongy substance is low. The intertrabecular space presents bone marrow reticular stroma with inclusions of adipocytes (yellow marrow). There is tough-fiber connective tissue to be seen in a small amount, which appears as inclusions. The trabecular bone matrix has not many yet regular bonding lines. The spongy bone features a network of interconnected, tightly adjacent bone trabeculae, while between them, in some areas, there could be adipose tissue identified against reticular connective tissue (red marrow). Bone beams have a flat surface with rare resorption lacunae without being replaced by osteoblasts; osteocytes can be visualized in the trabecular structures thickness. The following factors point at a slowdown metabolism and a decrease in mandible alveolar part osteohistogenesis: small and far-spaced bonding lines in bone trabeculae; areas in bone trabeculae with no bonding lines; an insignificant number of flattened, functionally inactive cellular elements in the endossal layer (Fig. 5).
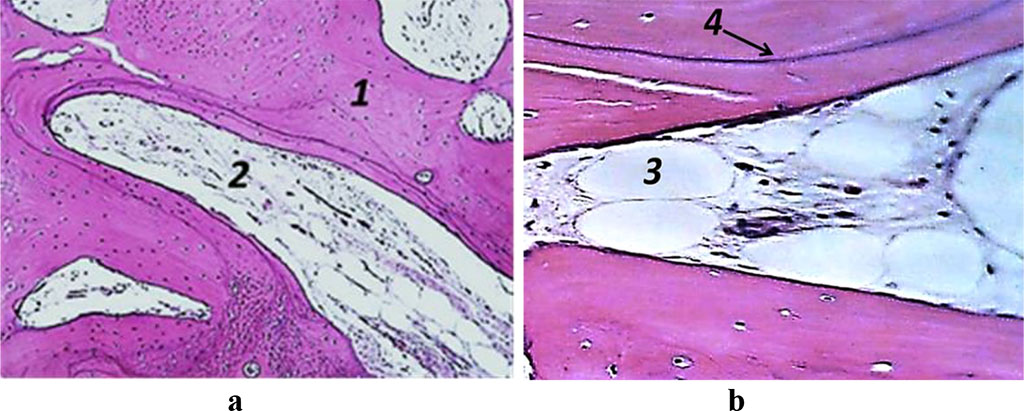
Figure 5. Histological preparation, Patient M., 53 y.o., hematoxylin-eosin staining; a – magnification (×250); b − magnification (×500); 1− bone trabeculae, 2 − red bone marrow, 3 – adipose tissue, 4 – resorption lacuna.
Therefore, in the control group, where the patients had no endocrine pathologies or bone remodeling issues in the mandible alveolar microarchitectonics at the area of missing teeth, there were some morphological changes observed, which means a positive bone balance, given the age-related decrease in the metabolism intensity.
Of the 24 patients in the main group, 3 (12.5%) had bounded dentition defects; 6 patients (25.0%) featured unilateral terminal defects; 10 (41.7%) – bilateral terminal defects; 5 (20.8%) patients had complete lack of teeth in the lower jaw. The CBCT data showed that of 24 patients of the main group, 7 (29.2%) had a sufficient volume of bone tissue at the dentition defect area (category A, classification by C.E. Mich, K.B.M. Judy (1987)), whereas another 17 patients (70.8%) had a minimal sufficient volume of bone tissue (category B, classification by C.E. Mich, K.B.M. Judy (1987).
The CBCT of the cross-sectional 3D window at the area of planned dental implantation in the main group revealed minor changes in the bone tissue microarchitectonics, while the amount of bone was maintained. The 3D window of volumetric rendering mode showed that at the area of dentition defects, the cortical bone was wide and intact throughout. There were some minor qualitative changes observed in the spongy bone: the spongy bone trabeculae width and the bone trabeculae structure density were slightly reduced; there were some spots identified where the intertrabecular space was expanded; the vestibular and lingual cortical plates were intact; the trabecular structure preserved its integrity yet there was a slight change in the structure arrangement (disorientation) observed in the bone trabeculae (Fig. 6-8).
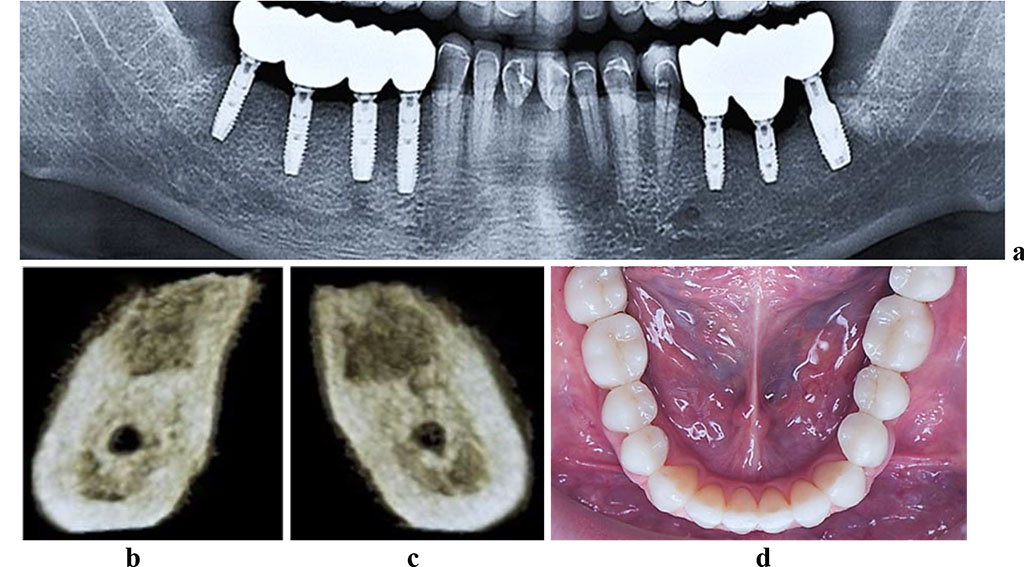
Figure 6. Patient K., 57 y.o. Diagnosis: lower jaw partial adentia; Class I by Kennedy. Lower jaw CBCT: a – bone tissue 12 months following the installation of dental implants (panoramic reformat); 3D cross-sections at the adentia area 3.6 (b) and 4.6 (c) prior to the installation of dental implants; d – lower dentition after implantological and prosthetic treatment.
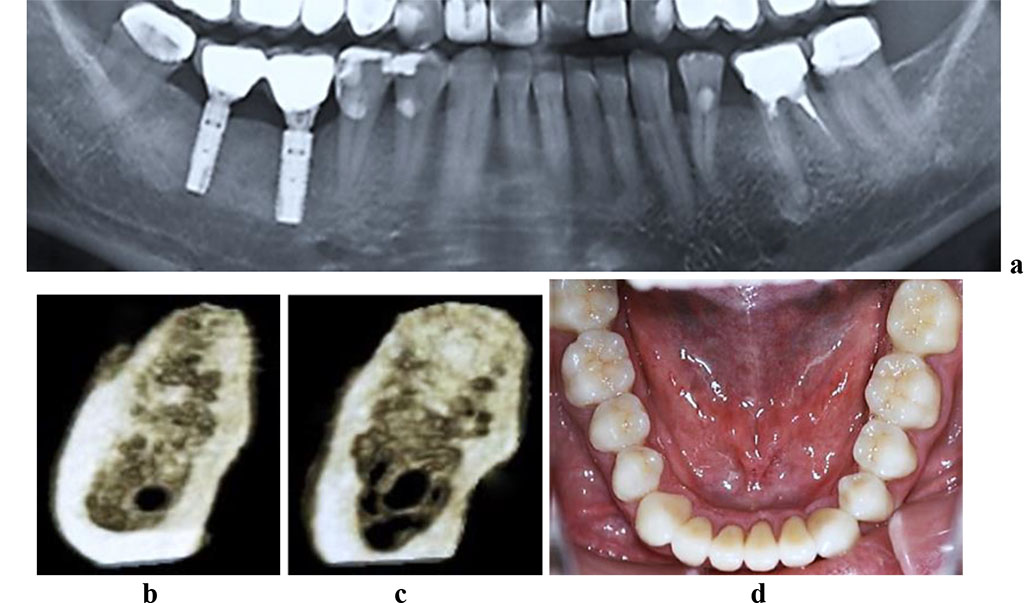
Figure 7. Patient R., 51 y.o. Diagnosis: lower jaw partial adentia; Class III by Kennedy. Lower jaw CBCT: a – bone tissue 12 months following the installation of dental implants (panoramic reformat); 3D cross-sections at the adentia area 3.7 (b) and 3.6 (c) prior to the installation of dental implants; d – lower dentition after implantological and prosthetic treatment.
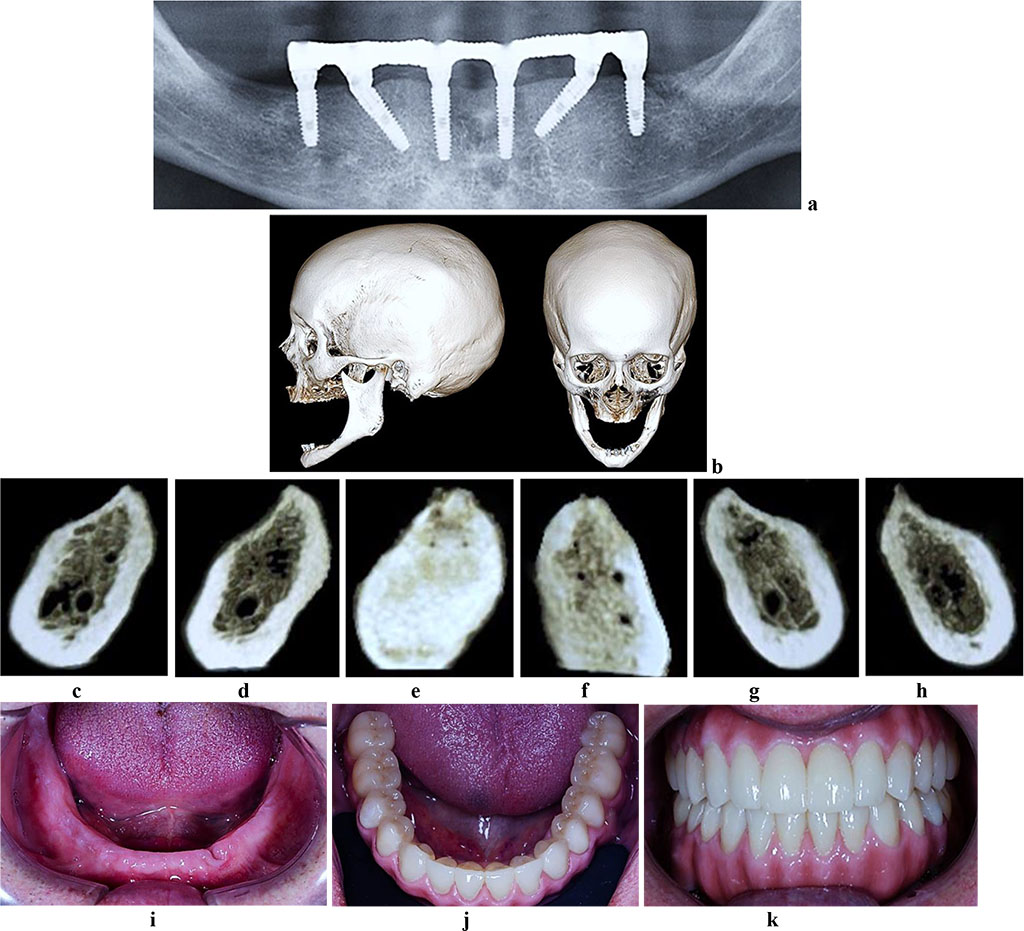
Figure 8. Patient S., 62 y.o. Diagnosis: lower jaw total adentia; Class I by Keller. Lower jaw CBCT: a – bone tissue 12 months following the installation of dental implants (panoramic reformat); b – volume rendering; 3D cross-sections at the adentia area 3.2 (c), 3.4 (d), 3.6 (е), 4.2 (f), 4.4 (g), 4.6 (h) prior to the installation of dental implants; lower dentition before (i) and after (j, k) implantological and prosthetic treatment.
The histological preparations of the lower jaw alveolar part bone tissue in the main group, taken at the adentia area, presented a cortical plate and partially spongy substance. The cortical plate had a lamellar structure with wide confluent Haversian canals. There were periosteum elements to be found on the outer surface of the cortical plate; the surface layers contained immature bone substance. The Haversian canals were of a rounded shape, filled with mature vessels. The inner surface was smooth.
The bonding lines were well developed, but not regular, their course varying, appearing in broken lines. The lacunae contained osteocytes. The contour of the cortical plate inner surface was smooth. The cortical plate and the spongy substance had clearly cut boundaries. The spongy substance trabeculae featured medium thickness and contained an extremely small number of Haversian canals. No osteoblastic activity was identified on the surface of the trabeculae and the inner lining of the Haversian canals. The spongy substance revealed no significant osteoclastic activity. The intertrabecular space was filled with bone marrow reticular stroma also revealing some inclusion of adipocytes (yellow bone marrow). Tough-fiber connective tissue, too, was to be found, while presenting as inclusions in small amounts. The bonding lines were well developed, yet not regular, with a varying course, manifested itself as broken lines. The lacunae contained osteocytes. As for the biopsy preparations obtained from the men of the main group, the following histomorphological features were to be observed at the adentia area: the spongy bone had a slight separation and thinning of the beam architecture (cortical plates) whereas the trabecular package was consistent; a slight decrease in the osteogenic pool cells in the endossal layer, required for reparative regeneration; an increased number of peritrabecular areas filled with adipose tissue; irregular and low-number bonding lines in interconnected bone trabeculae; areas of newly developed poorly mineralized osteoid; bone trabeculae surface featuring individual lacunae of resorption with some activated osteoclasts in them (Fig. 9-11).
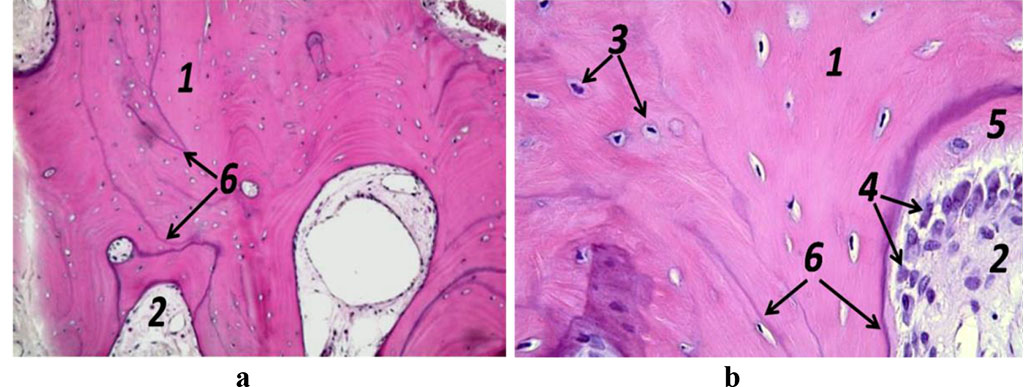
Figure 9. Histological preparation; patient K., 57 y.o.; hematoxylin-eosin staining; a – magnification (×250); b − magnification (×500); 1− bone trabeculae; 2 − red bone marrow; 3 – osteocytes; 4 – active osteoblasts, 5 – osteoid; 6 – bonding lines.
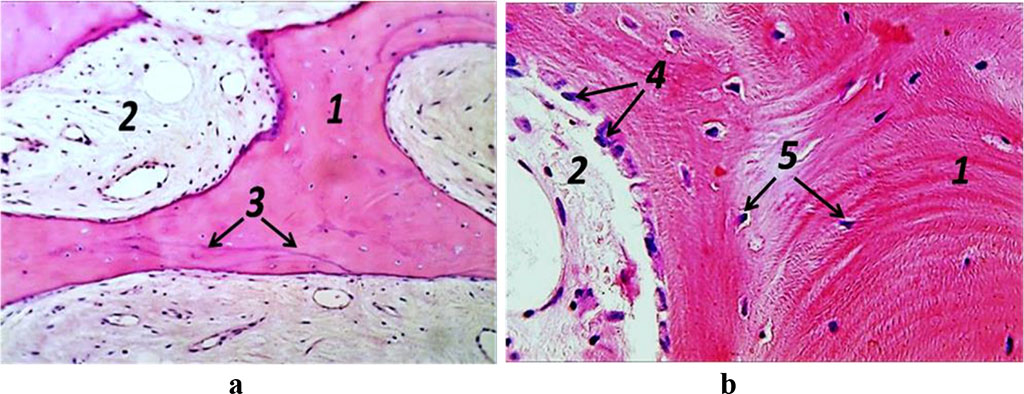
Figure 10. Histological preparation, patient R., 51 y.o.; hematoxylin-eosin stain; a – magnification (×250); b − magnification (×500); 1− bone trabeculae; 2 − red bone marrow; 3 – bonding lines; 4 – osteogenic cells; 5 – osteocytes.
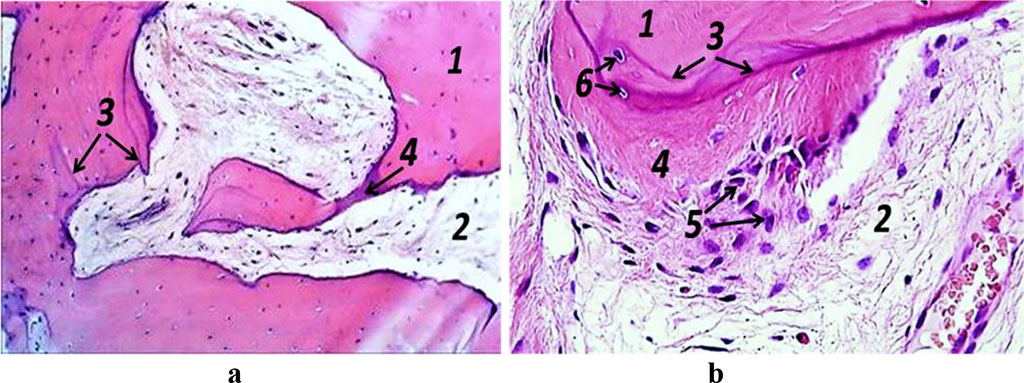
Figure 11. Histological preparation, patient S., 62 y.o.; hematoxylin-eosin staining; a – magnification (×250); b − magnification (×500); 1− bone trabeculae; 2 − red bone marrow; 3 – bonding lines; 4 – osteoid; 5 – osteogenic cells, 6 – osteocytes.
The above reveals that patients of the main group diagnosed with type 2 diabetes mellitus at the stage of carbohydrate metabolism compensation following pharmacological correction of the bone remodeling imbalance to the level of reference (normal) values, had their microarchitectonics of the lower jaw alveolar part (at the adentia area) showing some physiological restructuring (regeneration) and epithelialization of oral tissues, while the time required for osseointegration and healing appeared longer if compared to the control group.