- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.20
Pathological scarring at the present stage does not have a clear pathogenetic justification, it is characterized by unpredictability and constant growth of keloid scars, the absence of regression for many years and reasonable pathogenetic treatment. Despite the fact that there is the possibility of extirpation of excess tissue, it is almost impossible to remove keloid scars without recurrence. The paper presents phenotyping data in the structure of cellular ensembles of scars with the disclosure of signaling mechanisms of fibroblast differentiation into specialized cells that secrete collagen and the main matrix of the intercellular substance with pronounced hyalinization. It has been shown that the number of macrophages in the tissue of developing keloid scars is reduced in comparison with areas of normal reparative skin regeneration and in the structure of hypertrophic scars. Given the responsibility of macrophages for the secretion of angiogenesis factors and, accordingly, further oxygenation of the tissue healing zone, the authors point to a decrease in the number of macrophages as one of the main factors inducing keloid scarring and connective tissue metaplasia.
Keywords: hypertrophic scar; immune cells; inflammatory mediators; keloid; potential therapeutic targets; intercellular interactions, signal transduction pathways.
Despite the constant improvement of methods for the treatment of skin injuries of various origins and the tactics of postoperative management of patients, the formation of rough deforming scars is one of the serious problems of modern medicine [2]. Having appeared once, scars remain for life, while the issues of the biological essence of scarring, due to the lack of objective diagnostic and prognostic morphological criteria for the course of regeneration, are the subject of heated discussions [7]. At the present stage, the need to prevent excessive scarring after surgical interventions, injuries, etc. is an actual scientific and practical problem [15]. Despite the extensive discussion in the pages of the domestic and foreign press of the issues of pathogenesis, diagnosis and treatment of pathological scarring after operations and injuries, the opinions of specialists and scientists remain extremely controversial in determining common approaches to solving these issues [4]. Diagnosis of pathological scars does not present great difficulties and is based on anamnestic data and clinical manifestations [10]. Most of the described methods of treatment and prevention of excessive growth of connective tissue are related to patients who have formed keloid and hypertrophic scars [6]. Meanwhile, to date, there are no such methods of treatment that would reliably stop the progression of the pathological scar and its recurrence after surgical treatment [11]. Despite the fact that already in the early 80s. of the 20th century, the concept of skin-associated lymphoid tissue (SALT) was formulated, which continues to develop today, the totality of clinical and experimental data accumulated today does not allow one to obtain an exhaustive understanding of the important role of immunophagocytic cells in skin regeneration [8]. The available studies do not reflect the key pathogenetic factors in the development of keloid scars in the process of skin regeneration, and the lack of data on the histogenesis of scars at the present stage underlies ineffective methods for their treatment and prevention of relapses, which requires a thorough study of these issues.
To study the phenotypes of cell ensembles in the zone of reparative regeneration of the skin after injuries of various origins to identify their role in the formation of keloid scars.
The biopsy samples of keloid scars in 43 patients of the same age group after cosmetic surgeries (29) and burns (14) were studied. The skin of 3 patients after surgical operations not associated with skin lesions in the same age group was used as a control. The distribution of the material is presented in table 1 and reflected in Figure 1.
Table 1. Distribution of clinical material of skin with keloid scarring
| № | Skin biopsies of the control group | Skin of patients after burns | Skin of patients after cosmetic surgery |
| 1 | 3 | 14 | 29 |
*Note – data in absolute numbers.
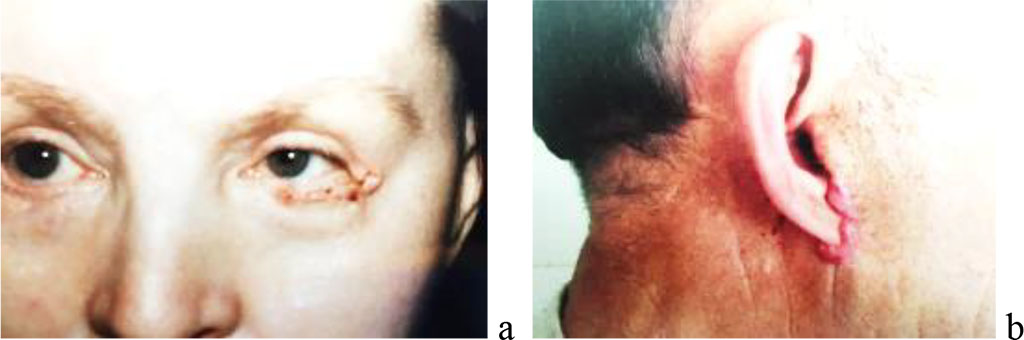
Figure 1. A) Eyelid surgery of a woman. B) Keloid scar in the area of the auricle of a man.
The study was conducted in accordance with the Helsinki Declaration, with the permission of the ethics committee of the Far Eastern Federal University. Along with the classical morphological methods of Van Gieson staining and hematoxylin and eosin, immunohistochemical methods were performed to detect the localization of CD4, CD8, CD68, CD163, CD34 positive cells, as well as VEGFa and VEGFb expression. The illustrations were made and analyzed using a Bx52 microscope and a PDx25 camera with proprietary software.
Results of own research The histological picture of normal human skin of different age groups is characterized by a well-defined papillary layer protruding into the epidermis, with good blood supply, basal epithelium with severe basophilia, and a dense stratum corneum (Figure 2).
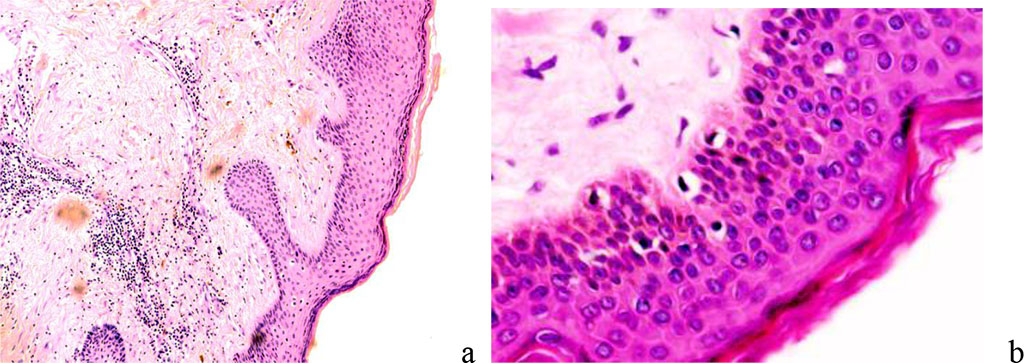
Figure 2 - normal skin. Stained with hematoxylin and eosin. Magnification A) x100; b) x400.
The composition of epidermal differons includes not only basal, spiny, granular cells, of which the first two pools represent the cambium, but also Langerhans and Grenstein cells, which provide cellular immunity, sensitive Merkel cells, melanocytes, as well as a small amount of intraepithelial lymphocytes. The dermis is represented by two layers, papillary and reticular. Collagen fibers of the papillary layer are of type III, type I collagen is characteristic of the reticular layer of the dermis. After alteration of the skin at the edges of the wound on the border with healthy tissue, an increase in the proliferative activity of keratinocytes begins, the daughter cells creep onto the surface of the injury and the wound closes. For several hours, there is a narrowing of the blood vessels in the injured area to prevent the release and loss of fluid. Platelets in the area of the wound surface are accompanied by the formation of thromboplastin, which causes blood clotting and promotes the formation of fibrin. At the same time, there is an intensive migration of formed elements from the vessels of the dermis, which is necessary not only for phagocytosis of necrotic tissues, but also for control of reparative regeneration (Figure 3).
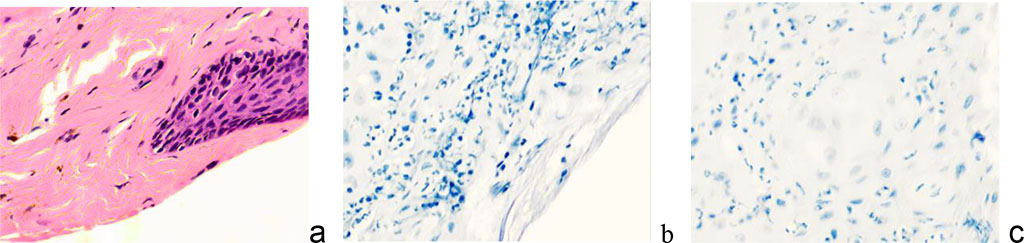
Figure 3 - Wound surface of the skin: a - wound edges, epithelialization of damage; b, c, d – infiltration of the damaged area by leukocytes. Magnification. x 100. Staining: a) with hematoxylin and eosin; b, c) hematoxylin.
The spiny layer in the norm and in scarring differs in that the boundaries between the cells in the scars are weakly expressed (Figure 4).
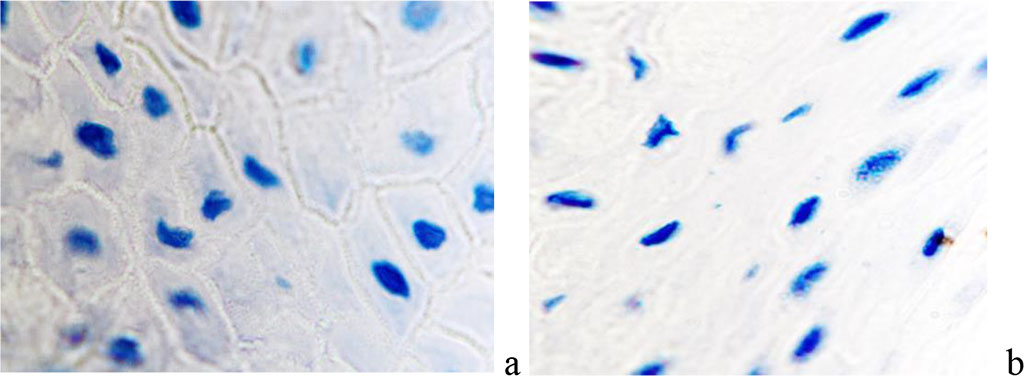
Figure 4 - The wound surface of the skin: a - the prickly layer of the epidermis is normal; b: spiny layer of the epidermis with scarring - intercellular boundaries are not identified. Magnification x 200. Staining with hematoxylin.
We have stated that under thermal skin injury the reparative regeneration and the closure of the wound surface occur due to epithelial restitution in the direction of the burn injury. At the same time the defect is closed on behalf of hair follicles that migrate into the wound site. (Figure 5).
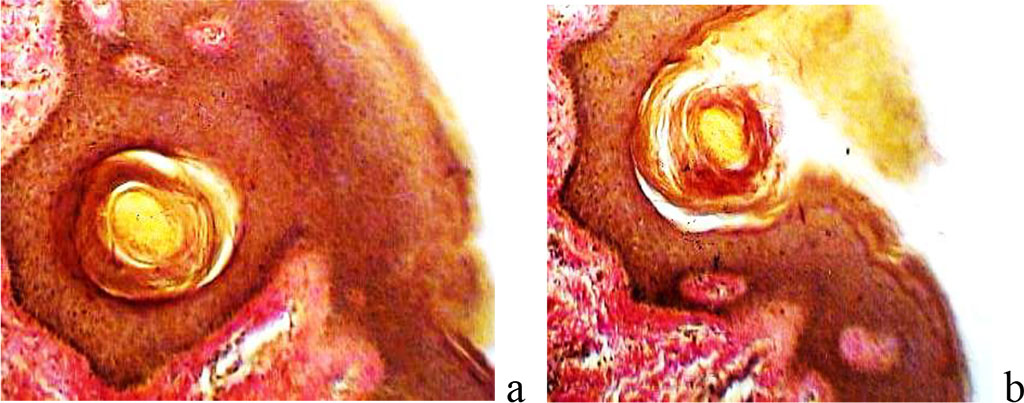
Figure 5 - Closure of the wound surface in the area of epidermal necrosis due to the exit of the hair follicle to the surface. Coloring according to Van Gieson; Magnification x100.
When the bottom of the follicle reaches the surface of the damaged area, the epidermis of the skin acquires a structure corresponding to functional needs, the healing process ends with complete regeneration, and not only with the restoration and epithelialization of the defect, but also with the performance of the function.
According to our observations, if horizontal growths of keratinocytes occur under necrotic tissue, the latter will be rejected from the surface of the emerging epithelium due to the release of monocyte-macrophages to the wound surface. When the skin is damaged to a great depth, below the basement membrane and the location of melanocytes, after repair of the skin defect, atrophic skin or a depigmented spot develops. Injuries to the skin below the scallops of the epidermis at the border of the papillary and reticular layers of the dermis end in scarring. In the presence of skin appendages, even if they are damaged and their structure is disturbed, complete regeneration is possible, although with the formation of a scar, but covered with normal thin skin (Figure 6).
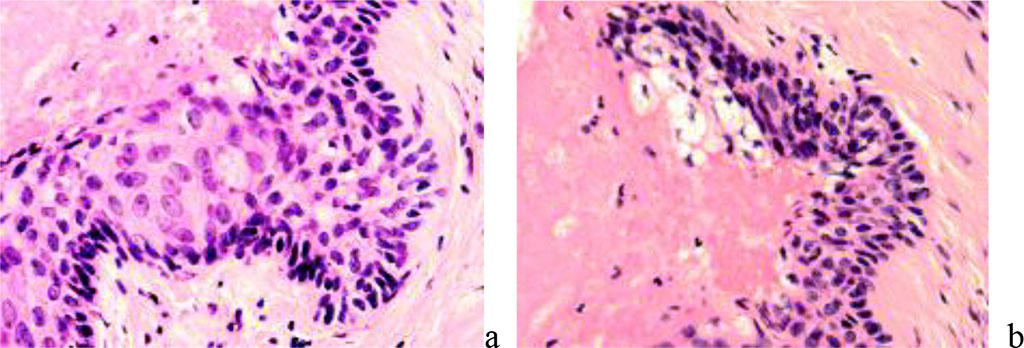
Figure 6 - reparative regeneration of the skin with the germination of the epidermis under the necrotic wound area. Staining: a) with hematoxylin and eosin. Magnification x200.
Regeneration with the formation of a papillary layer protruding into the epidermis with vascularization of vertical capillary networks indicates normal regeneration without scarring, but if the trophic supply of the skin is not restored, rough scarring of the skin occurs. In this case, the relief of the papillary layer is disturbed, there is no vascular network corresponding to healthy, intact skin. In the process of regeneration, there is a change in the number of leukocytes infiltrating from the vessels, after the inflammatory and proliferative stages in the dermis, a decrease in the number of cells, a decrease in proliferative activity, a change in the ratio of cells and intercellular substance in the direction of increasing the specific gravity of the latter both in the papillary layer and in the reticular layer. We have noted that the course of growing capillaries in the regenerating skin of keloid scars is disturbed, the direction of collagen fiber bundles does not provide a looped structure of the fibrous network, but more often represents parallel bundles of collagen fibers.
Not only the reticular layer, but also the papillary layer acquires the features of a dense, formed connective tissue, poor in cellular differons, most of which are fibroblasts. If melanocytes are detected on sections, the scar may have a color identical to the surrounding skin. In the absence of melanocytes, the scar is white and distinct from the surrounding skin. With the preservation of the vasculature, the color of hypertrophic and keloid scars is red, since the vessels are located in the thinned dermis and are surrounded by a thin layer of the epidermis (Figure 7).
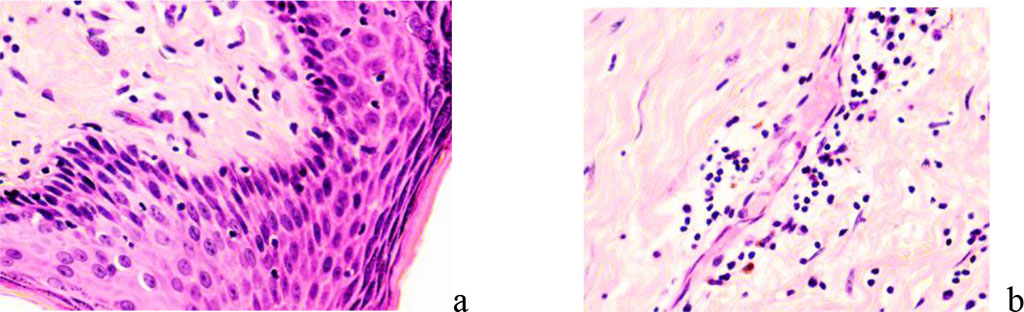
Figure 7 - Papillary and reticular layers of the dermis under conditions of reparative regeneration of the skin in the neck area: a - regeneration area of a hypertrophic scar; b – area of keloid scar formation. Stained with hematoxylin and eosin. Magnification x 200.
The epidermis may include forms that are not typical of the epidermis. There are vacuolar degeneration, smoothing of the papillae, an increase or decrease in the number of melanocytes, the stratum corneum is often thinned. In the dermis, the number of histions with pronounced zones of growth and pericapillary transformation of fibroblasts is increased. A change in the ratio of cells and intercellular substance with the advantage of the latter in the connective tissue of the dermis, especially in the papillary layer, indicates a rough keloid scarring. Orderliness is observed in the direction of collagen bundles, in contrast to the data of other authors. The beam thickness reaches 120 nm. Appendages of the skin disappear, both hair follicles and sweat glands are absent (Figure 8).
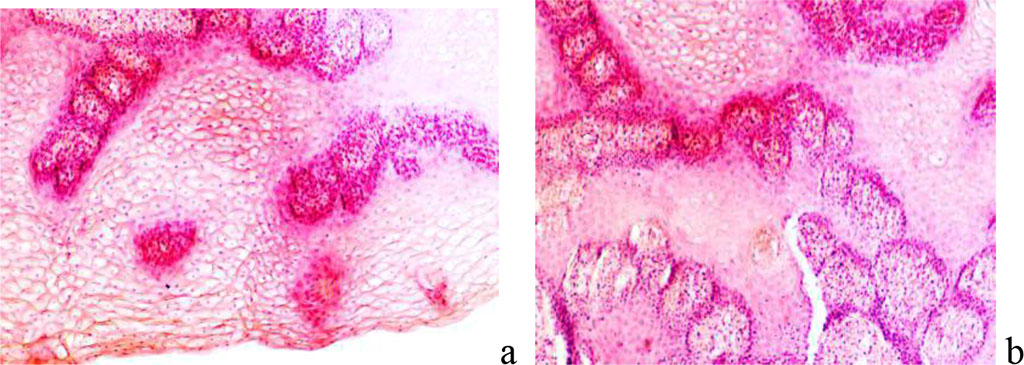
Figure 8 - Epidermis of the skin with keloid scars: a - earlobe; b – breast skin after correction. Stained with hematoxylin and eosin. Magnification x 200.
Classically, keloid scars in their development go through a cascade of complex, dynamic and overlapping processes, consisting of inflammatory, proliferative and remodeling reactions [12]. The stage of epithelialization begins after injury, the damaged area is covered with a thin epithelial film, which thickens, coarsens, becomes pale in color within 7–10 days and remains in this form for 2–2.5 weeks. At the stage of swelling, the scar increases, rises above the adjacent skin, becomes painful. Over the course of 3–4 weeks, the pain sensations subside, and the scar acquires a more intense reddish color with a cyanotic tint. At the next stage, the scar thickens, dense plaques appear in some of its places, and the surface becomes bumpy. The external picture of the scar is a keloid, which is characterized by the growth of scar tissue beyond the edges of the damaged area of the skin. At the stage of remodeling, the scar finally acquires a keloid character. It is distinguished by its pale color, softness, mobility and painlessness, and its regression can last for many years [3]. In the phase of epithelialization, the skin is restored with the formation of a scar, the final healing of the wound. Cell populations of keloid scars are represented by numerous fibroblasts, among which the content of atypical giant fibroblasts is significant, the number of plasma cells and lymphocytes is reduced. Fibroblasts tend to symplast formation. In the area of damage and the underlying upper layers of connective tissue, cells appear that are responsible for the restructuring of the skin. Monitoring showed that CD8 and CD163 positive cells, macrophages, fibroblasts, and mast cells are identified at this stage (Figure 9).
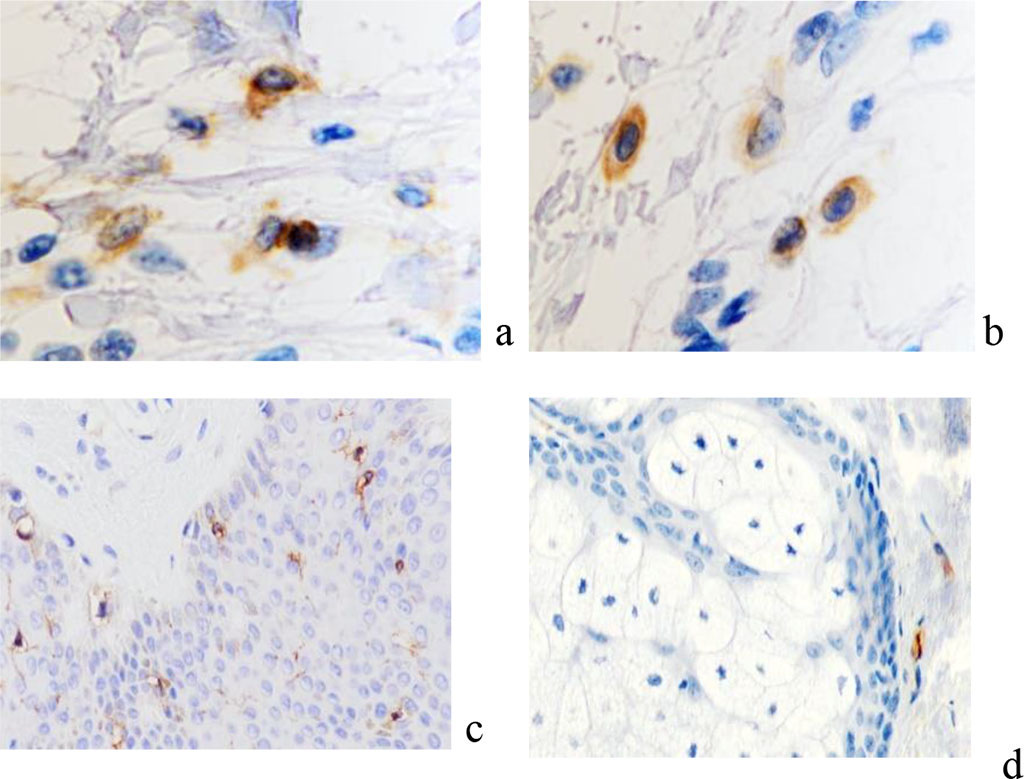
Figure 9 - Localization of CD in the skin of patients of the observation group with keloid scars in the auricle area. Immune histochemistry to detect the localization of CD cells (marked with arrows): a - CD4; b - CD8; c – CD68; d – CD163; e - CD203a; c, d, e – magnification x 200; a, b magnification x 400.
There is a decrease in the number of macrophages in the area of keloid scarring compared with the areas of formation of normotrophic scars. Since in the third phase it is necessary to provide maximum protection of the skin wound from external influences in order to promote full regeneration, CD8+ lymphocytes appear that produce the same cytokines as CD4+-Th1 (TNF-alpha and beta, IL-2.3, gamma- IF, GM-CSF). These immunocytes limit the immune response, preventing hypersensitivity reactions and triggering autoimmune processes. The data obtained by us are the result of the fact that in the acute stage of damage to skin structures as a result of activation of the parasympathetic system, the terminal arterioles expand, blood perfusion begins along the capillary bed and the migration of effector cells of the immunophagocytic link into the damaged tissue. But at the same time, a massive exit into the bloodstream of the decay products of necrotic tissues from the affected area begins. This causes the phenomena of endotoxicosis, which negatively affects both the vascular wall of capillaries and arterioles, and its permeability. As a result, the number of functioning capillaries, although higher than in the alteration stage, is nevertheless significantly lower than normal values. In keloid scars of the skin of the auricles and earlobes, the distribution of immunocytes differs from the data obtained on skin burns of various localization and skin damage due to surgical operations accompanied by the formation of keloid scars.
We noted that in the keloid scars of the skin of the earlobes and auricles, the formation of a collagenous-fibrous skeleton and the acquisition of spindle-shaped epidermis by keratinocytes not only in the upper layers, but also in the basal ones are noted, while the location of the cells is characterized by a strictly ordered direction in parallel rows in accordance with the surface of the epidermis. Macrophages and mast cells are also located parallel to the surface of the epidermis and are identified at the basement membrane, thickened up to 100 microns and having a heterogeneous structure throughout, the location of fibroblasts is similar to their distribution in collagenous fibrous cartilage (Figure 10).
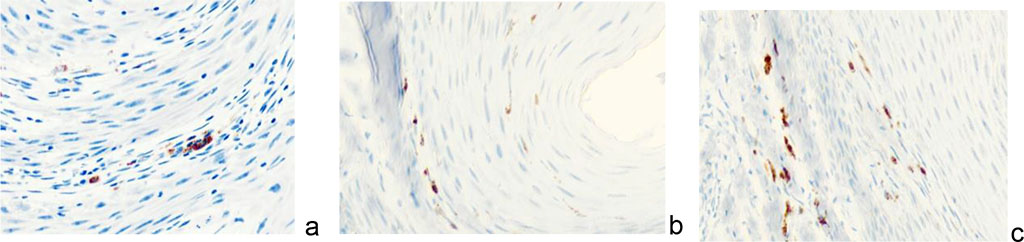
Figure 10 - Immunocytes CD in the structure of keloid scar in the auricle area of a 43- year-old man. Immunohistochemistry used to detect localization of immunocytes in the skin of the auricle. Magnification: a, b) х100; a, b, c) х 200
Our studies have established that the number of endotheliocytes in the structure of existing and newly formed vessels containing receptors for VEGF proteins, which are activators of angiogenesis, increases under scarring conditions, which is associated with an increase in the physiological demands of growing scarring tissue. It is known that VEGF proteins exist within the system responsible for providing oxygen supply to tissues against the background of insufficient blood circulation. One of the main functions of VEGF is the induction of angiogenesis, the formation of new blood vessels in case of damage, the provision of collateral circulation to create new vessels in the event of destruction of existing ones. These facts can be used in the development of new strategies for the treatment of keloid scars and keloid disease through the inhibition of tissue growth by turning off the blood circulation that is adequate to the physiological needs of the newly formed tissue. At the same time, the effect on VEGF receptors, and not on protein synthesis by macrophages, is more prognostically effective. In our studies in growing keloid scars, a high reliability of an increase in CD34 expression was noted, which is identified both in the wall of newly formed and growing vessels, and in the stroma of the scar tissue (Figure 11).
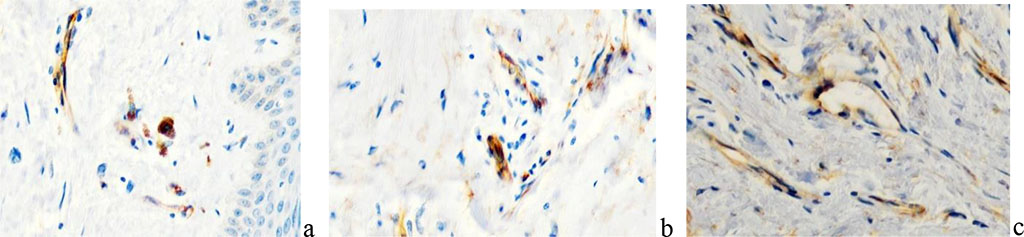
Figure 11 - Localization of cells expressing VEGF in the scar structure. immune histochemistry. Magnification a, b, c x200.
VEGF receptors are detected not only in the endothelium, but also in fibroblasts, which indicates that collagen-producing cells can be targets for the suppression of synthetic function by inhibitors of endothelial growth factors synthesized by macrophages and lymphocytes. VEGF regulates almost all endothelial cell functions necessary for capillary formation: migration, proliferation, differentiation, and survival of endothelial cells; therefore, it may be one of the key factors in the development of cell technologies in the treatment of keloid disease.
According to our data, the number of capillaries in keloid scars at the stage of inflammation is higher than in healthy skin, and also during the formation of hypertrophic scars, the subsequent relative decrease in the total area of the vascular bed may be associated with edema, leukocyte infiltration of the inflamed tissue. Lee J.H., Ji S.T., Kim J., Takaki S., Asahara T., Hong Y.J., Kwon S.M. (2016) noted that the number of new vessels affects the survival of transplanted cells and a decrease in the migration of cytotoxic T cells, macrophages and neutrophils, causing activation of fibroblasts in the damaged area in the remodeling phase [5]. Direder M., Weiss T., Copic D., et al. (2022) identified Schwann cells in the structure of keloid scars, which confirms the hypothesis about the role of keloid Schwann cells with differentiation inhibited by macrophages in the formation of the extracellular matrix [2].
Given the fact that the p53 protein is activated when the cell genome is damaged, triggering apoptosis, the lack of expression of this protein in the material of patients with the formation of keloid scars after skin lesions indicates that true keloid scars have common features with tumor processes, in which 50% p53. It is known that the p63 gene is required for committing epidermal stem cells in embryonic development. At the same time, p63 provides for the implementation of many functions that are necessary for the self-maintenance of epidermal stem cells of the definitive epidermis. Expression of p63 provides adhesion of keratinocytes and inhibition of apoptosis, as well as maintaining the integrity of the epidermis as a tissue. In our studies, a high expression of the p63 protein is noted in the scar tissue. A positive reaction was identified not only in the basal layer of the epidermis, but in all rows of keratinocytes of the spiny layer, this protein is expressed (Figure 12).
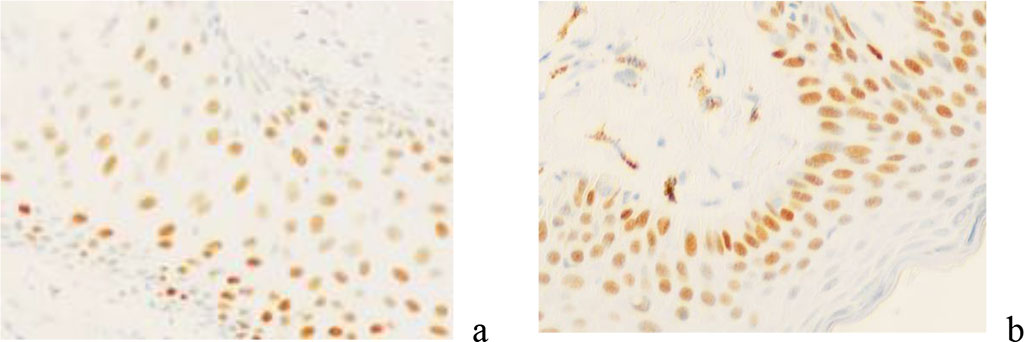
Figure 12 - Skin of a keloid scar in a 58-year-old woman. immunohistochemistry for detection of p63 protein expression. Magnification x 200.
Immune cells, signaling molecules, soluble inflammatory mediators, and their associated intracellular signaling pathways are critical regulators of inflammation and are involved in inducing and inhibiting healing of damaged skin with scar formation. One of the main factors inducing the normal course of the inflammatory process are macrophages secreting VEGFa and VEGFb, an inducer and inhibitor of angiogenesis.
A decrease in their number leads to a decrease in the growth of blood vessels in the zone of reparative regeneration, followed by insufficient oxygenation of differentiating cells with a vector of specialization for the secretion of intercellular substance with pronounced hyalinization. At the same time, the cells retain the properties of stem mesenchymal fibroblasts, with high proliferative activity and a stable genome. Additional confirmation of the predominance of proliferative processes and a decrease in differentiation processes was obtained using methods for detecting the expression of p53 and p63 proteins. Analysis of the data obtained showed that apoptosis in damaged skin is reduced, and due to increased tissue renewal, the migration of daughter cells to the surface layers, the expression of the p63 protein increases and is identified in a larger number of rows of keratinocytes in all layers of the skin compared to changes in regenerating skin with normal healing. ncreased expression of CD34 not only in the endothelium, but also in the stromal cells of the connective tissue of scars is the result of an increase in the number of cells with receptors for endothelial growth factors VEGF. It is known that CD4 receptors are expressed mainly on T-lymphocytes and, being complementary to HLA class II antigens, provide contact of T cells with the macrophage-histiocytic microenvironment, on the cells of which HLA class II is expressed. The interaction of CD4 with HLA class II during intercellular contacts promotes the association of CD4 and the T-cell receptor into a single complex and the subsequent activation of the proliferative and secretory response of T-cells [9]. In general, we noted that in the process of wound healing in the first phase of healing, perivascular infiltration of CD cells is observed, which persists in the second phase of regeneration, and rarefaction compared to normal healthy skin. There is a marked activation and infiltration of CD4+ T memory cells into the wound surface area during the first post-injury period of inflammation. There is also an increase in the number of antigen-presenting cells - Langerhans cells (LCs), epidermal inflammatory dendritic cells (IDEC) and macrophages. Mast cell degranulation is observed, as in the studies of other authors [3, 14]. The regenerative process in the skin after damage is accompanied by its alteration and remodeling, the number of CD68 and CD163 cells increases and then decreases, which is caused by chronic inflammation. These skin changes are accompanied by thickening and increased collagen deposition in the dermis, which has been observed by other investigators [5,9]. By the end of the epithelialization phase, the skin is restored with the formation of a connective tissue scar, the final healing of the wound. Therefore, cells responsible for the restructuring of the skin appear in the zone of the burn wound and the underlying connective tissue layers. Monitoring showed that CD8 and CD163 macrophages, fibroblasts, and mast cells are identified at this stage. Since in the third phase it is necessary to provide maximum protection of the wound from external influences in order to promote full regeneration, CD8 + lymphocytes appear that produce the same cytokines as CD4 + (TNF-alpha and beta, IL-2.3, gamma-IF, GM -CSF). These immunocytes limit the immune response, preventing IS hypersensitivity reactions and triggering autoimmune processes. Ogawa R. (2017) suggests using therapies that reduce inflammation: injections dressings of corticosteroid ointment, radiation therapy, cryotherapy, compression therapy, stabilizing therapy, 5-fluorouracil (5-FU) therapy and surgical methods that reduce skin tension [9]. However, macrophages, endotheliocytes, fibroblasts are new therapeutic targets in solving the problem of inducing the healing process without the development of keloid scars through the effect on cell differentiation. Barallobre-Barreiro J., Woods E., Bell R.E., et al. (2019) identified 23 proteins that are unique exclusively to keloids, which is associated with impaired cell differentiation and specialization [1]. In the pathogenesis of keloid scars, a decrease in vascularization and hypoxia of the regenerating tissue are important, which contribute to the preservation of fibroblast stemness, continuous growth of keloid scars, inhibition of differentiation and hyalinosis of the intercellular matrix. The data obtained do not confirm the concept of Tan S., Khumalo N., Bayat A. et al. (2019), Ud-Din S., Bayat A. (2020) about the parallels between keloids and cancer [12, 13], but reveal the mechanism of metaplasia tissues due to a decrease in the number of macrophages and secretion of angiogenesis inducers, an increase in cells with VEGF reception, as well as trophic provision and tissue oxygenation, insufficient for the differentiation of mesenchymal fibroblasts into specialized ones with the loss of stem cell properties. This confirms the prospects for reversibility and regression of keloid scars through the creation of adequate conditions and trophic provision of the tissue after damage, compliance with all factors for successful reparative regeneration.