- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.17
Hantavirus infection is increasingly widespread in the last years, but diagnosing this infection could be difficult sometimes, given the nonspecific symptoms and the lack of experience by physicians into this direction. Hantavirus infection is a viral infection causing two major syndromes: Hemorrhagic fever with renal syndrome (HFRS) and Cardiopulmonary syndrome (CPS). We present the case of a young man, admitted to the hospital with fever, nausea, vomiting, diarrhea, abdominal pain and oligoanuria, in whom the diagnosis of hantavirus infection was established following the kidney biopsy, in addition to the ELISA assay. According to the literature, all histological findings concluded to hantavirus nephritis. Interstitial hemorrhage in the medulla is a main characteristic of hantavirus infection. Despite the fact that the identified serotype (Hantaan virus) is responsible for a severe form of this disease, the patient's evolution was favorable, with recovery of renal function after 1 month.
Keywords: Hantavirus infection, fever, kidney biopsy, interstitial hemorrhage, proteinuria
Hantavirus is an RNA virus from the Hantaviridae family, identified as pathogen for the first time in 1978 by Lee and al. Nowadays, over 30 serotypes have been identified, with Haanta, Dobrava and Puumala being the most common. Hantavirus is the etiological agent of: a) hemorrhagic fever with renal syndrome (HFRS) also called reno-hemorrhagic syndrome (associated with acute renal failure, proteinuria, hematuria and thrombocytopenia) and b) pulmonary syndrome -HCPS (severe pulmonary edema, rarely impaired renal function) [1]. While Puumala serotype causes the mildest form of HFRS, Dobrava causes the most severe clinical manifestation. Hantaan (HTNV) and Dobrava (DOVs) serotypes cause a severe form of HFRS in Asia and Southeastern Europe, with a mortality between 5–15%, while Puumala (PUUV) serotype cause a milder form of HFRS in Northern and Central Europe, with a mortality under 0.1%. The rodents carry this virus and the infection is transmitted to humans by inhaling aerosols from urine, feces, secretions or saliva from infected rodents or through their bite. Patients with possible contact with mice or other rodents (especially people from rural areas, camping trips), that present with the triad: thrombocytopenia, fever and acute kidney injury, can be suggestive of hantavirus nephritis. Proteinuria and hematuria are very common in hantavirus infection. Proteinuria is non-selective and can be massive – of nephrotic range ( > 3,5 g/24h) [2,3].
In terms of pathogenesis, the main target of the virus is endothelial cells; the destruction of
these cells leads to capillary leakage and dysfunction of glomerular filtration. In severe forms of the disease, overproduction of proinflammatory cytokines (“cytokine storm”) plays a key role in the pathogenesis of the disease. Disease severity has been associated with concentrations of IL-6, IFN-gamma, TNF-alpha, IL-18.
A 30 year-old man coming from rural areas (Buzau region, Romania), without any medical history, but with recent domestic exposure to toxic substances (paint and varnishes) and coming from a recent fishing trip where he slept 1 night in a tent, is admitted to Nephrology department at Buzau Hospital.
The patient initially presents to the Emergency Room of the local hospital for malaise, fever, nausea, vomiting, diarrhea, abdominal pain and oligoanuria. The symptoms started with 5 days before the hospital presentation and the patient's received antibiotics without medical approval, but without the improvement in the symptomatology.
The results of the blood tests showed a high serum creatinine level (from 4 mg/dl to 8 mg/dl in 24-48h) and a high level of blood urea nitrogen (BUN), severe thrombocytopenia (PLT=2000/mm3), leukocytosis and inflammatory syndrome (fibrinogen=769 mg/dl; PCR=180 mg/l). Thus, it is decided to transfer the patient to the Nephrology department of Fundeni Clinical Insitute. Physical examination reveals: bad general condition, no fever, skin and mucous membranes discreetly pale, no bruising, no edema, blood presure of 145/90 mmHg, pulse 67 b / min, tender and painful adomen at touch, especially in the hypogastrium, 2-4 stools / day, diarrhea, urine output < 400 ml / 24h, hematuric urine. Blood test shows (Clinical Institute Fundeni): high levels of serum creatinine and blood urea nitrogen, hypoalbuminemia, leukocytosis, severe normochromic normocytic anemia, thrombocytopenia, inflammatory syndrome and a low level of C3 component. Urine test shows: hematuria and important proteinuria (18 g/l). CT examination of the chest, abdomen and pelvis shows: massive pleurisy on both sides, small amounts of fluid in the peritoneal cavity, normal size and position of the kidneys, without hydronephrosis.
Given the severe renal impairment with oligoanuria, severe thrombocytopenia, hemolytic anemia, abdominal pain and diarrhea, the high suspicion of the diagnosis was secondary PTT versus SHU secondary to an infection. An analysis was further used - the determination of ADAMTS-13, which was found to have normal activity, so the diagnosis of secondary PTT can be excluded.
The patient's condition and blood tests did not improve although he received pulse corticotherapy IV, so, an explanation and treatment measures had to be found quickly. The only action that could bring additional information was a kidney biopsy, which was done (Fig. 1; Fig. 2, Fig. 3, Fig 4. and Fig. 5).
The treatment consisted in: corticotherapy iv, parenteral fluids, temporary hemodialysis and antibiotics, with a good clinical and biological response after the treatment.
So, after 4 weeks of treatment, serum creatinine is normal, thrombocytes are normal and proteinuria is absent.
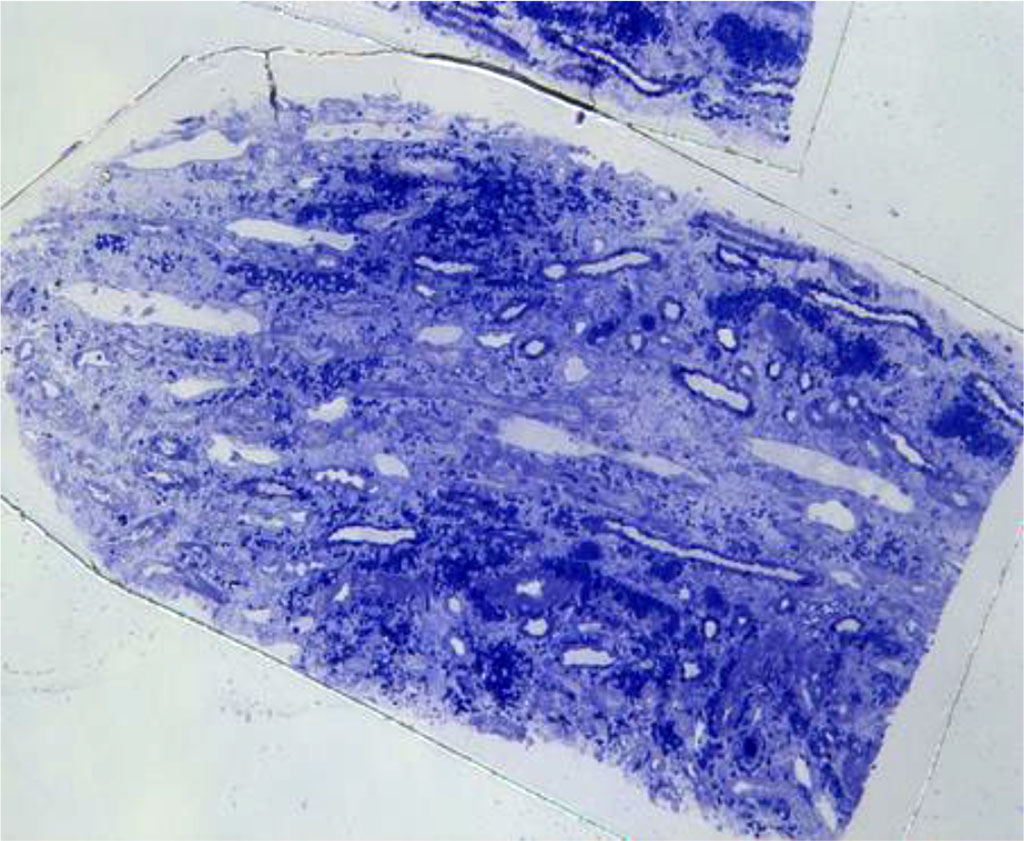
Figure. 1 Light microscopy- Severe interstitial hemorrhage in the medulla without interstitial inflammatory reaction
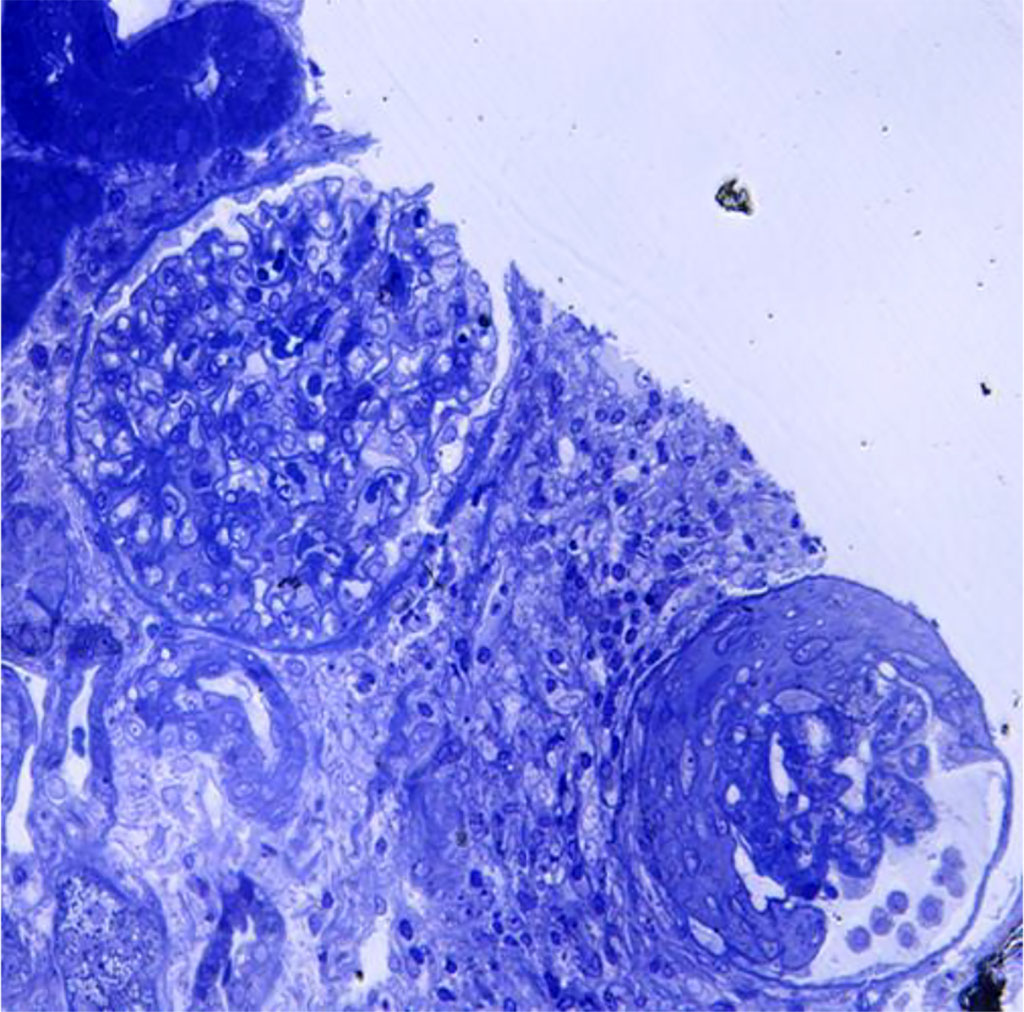
Figure 2. Light microscopy- glomerular sclerosis; periglomerular lymphoplasmocytic inflammation
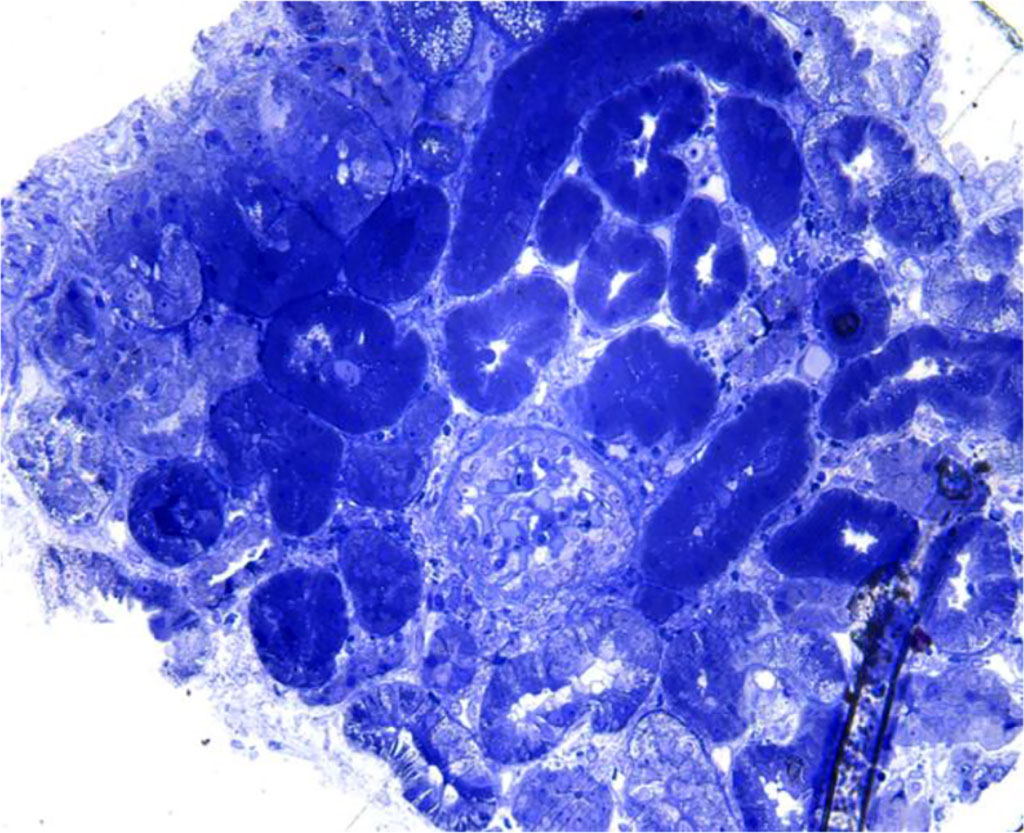
Figure 3. Light microscopy – absence of interstitial hemorrhage in the renal cortex
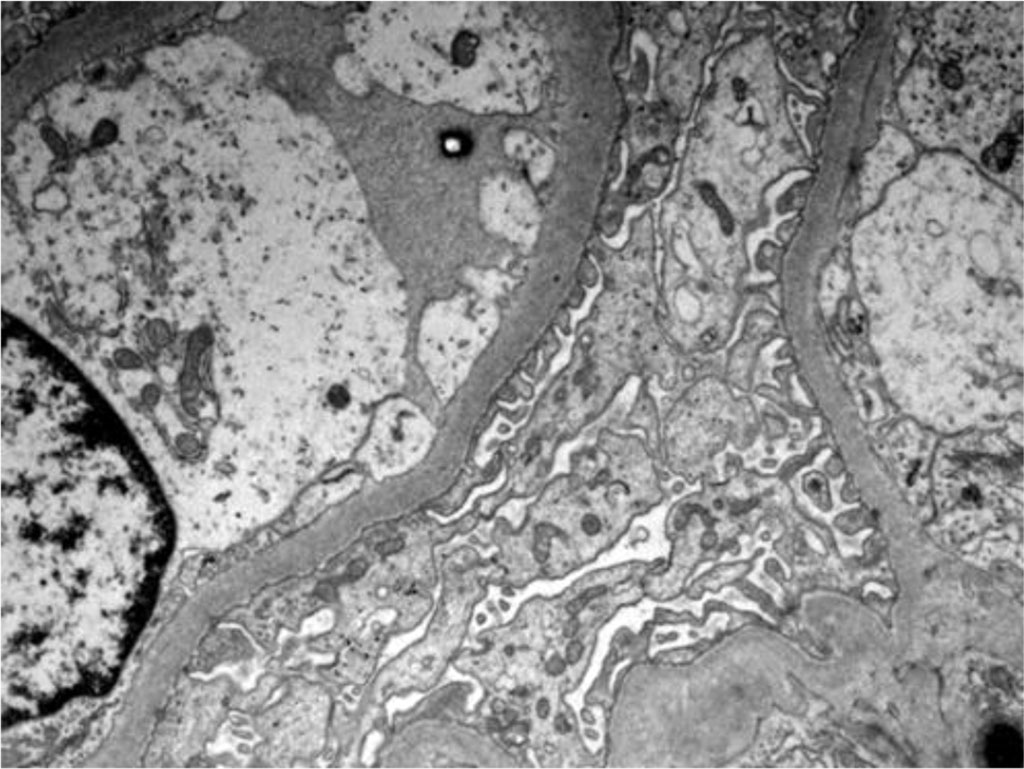
Figure 4. Electron microscopy - Podocytes foot process effacement
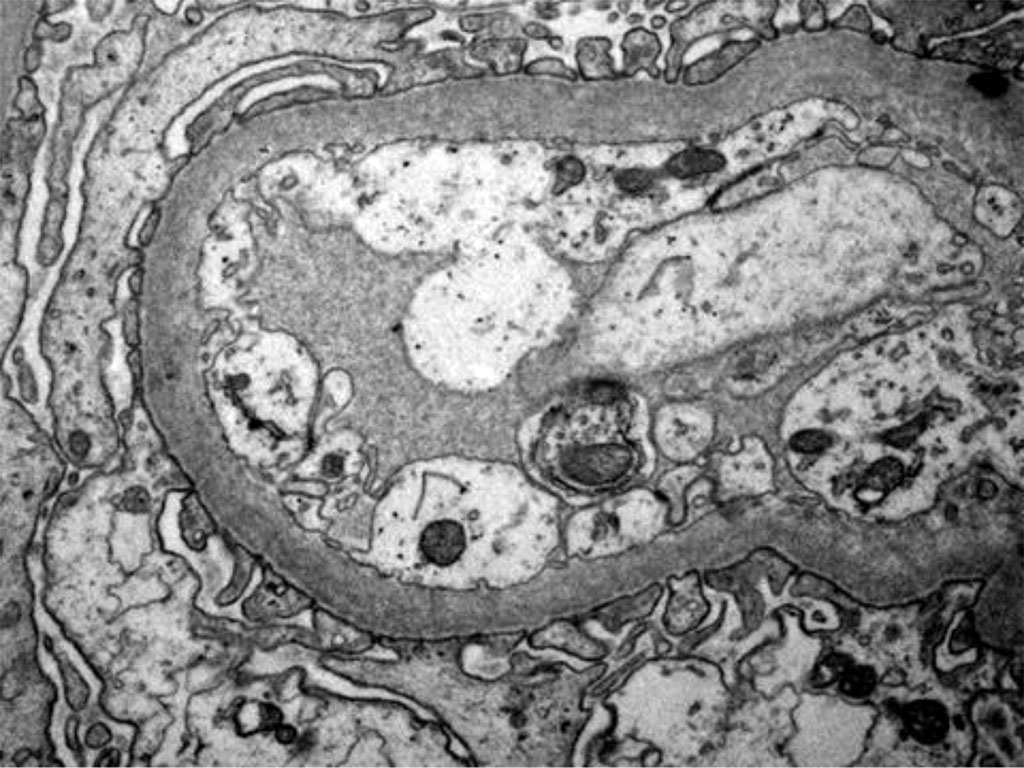
Figure 5. Electron microscopy - Podocytes foot process Effacement
Table 1. Cases of renal hemorrhage
| Situation | Cortex hemorrhage | Medulla hemorrhage |
| Viral infections (ex: hantavirus, leptospira, rickettsia, herpes simplex virus) | NO |
YES |
| Acute renal transplant rejection | YES | YES |
| Renal infarction, renal vein thrombosis | YES |
YES |
| Complications of invasive procedures (renal biopsy) | YES |
NO |
Renal biopsy is an invasive technique that is done to establish the exact diagnosis and choose the right treatment. In our case, the renal biopsy gave us an important information: interstitial hemorrhage in the medulla, which is highly suggestive for hantavirus nephritis. Renal hemorrhage can be found in some different situations (Table 1) [1].
Returning to the case of our young man, we can exclude acute transplant rejection because the patient is not transplanted; a renal infarction or renal vein thrombosis would have been objectified on the CT examination of the abdomen and pelvis; and a post-procedural complication would have been highlighted following the ultrasound control performed 24 hours after the renal biopsy. Thus, the hemorrhage encountered in the renal medulla and not in the cortex, directs our diagnosis to a viral infection.
Hantavirus is an RNA virus from the Hantaviridae family, identified as pathogen for the first time in 1978 by Lee and al. Nowadays, over 30 serotypes have been identified, with Haanta, Dobrava and Puumala being the most common. While Puumala serotype causes the mildest form of HFRS, Dobrava causes the most severe clinical manifestation. Hantaan (HTNV) and Dobrava (DOVs) serotypes cause a severe form of HFRS in Asia and Southeastern Europe, with a mortality between 5–15%, while Puumala (PUUV) serotype cause a milder form of HFRS in Northern and Central Europe, with a mortality under 0.1%. Sin nombre (SNV) and Andes (ANDV) serotypes are the agents of HCPS in Northern and Southern America, with a mortality ~40% [1].
The rodents carry this virus and the infection is transmitted to humans by inhaling aerosols from urine, feces, secretions or saliva from infected rodents or through their bite and can cause mortality in 5-15% of the cases [2].
Hantavirus is the etiological agent of: a) hemorrhagic fever with renal syndrome (HFRS) also called reno-hemorrhagic syndrome (associated with acute renal failure, proteinuria, hematuria and thrombocytopenia) and b) pulmonary syndrome -HCPS (severe pulmonary edema, rarely impaired renal function) [3].
Patients with possible contact with mice or other rodents (especially people from rural areas, camping trips), that present with the triad: thrombocytopenia, fever and acute kidney injury, can be suggestive of hantavirus nephritis.
Proteinuria and hematuria are very common in hantavirus infection. Proteinuria is non-selective and can be massive – of nephrotic range ( > 3,5 g/24h) and that indicates a defective glomerular barrier. In a german study, proteinuria without hematuria at the time of diagnosis is a significant predictor for developing severe AKI in PUUV infections (Puumala virus) [4,5].
In terms of pathogenesis, the main target of the virus is endothelial cells; the destruction of these cells leads to capillary leakage and dysfunction of glomerular filtration. Innate and acquired immunity are activated and “fight” to remove the infection. In severe forms of the disease, overproduction of proinflammatory cytokines (“cytokine storm”) plays a key role in the pathogenesis of the disease. Disease severity has been associated with concentrations of IL-6, IFN-gamma, TNF-alpha, IL-18 [6].
The first cases in Romania of hemorrhagic fever associated with renal syndrome were described in 1976 and included 11 patients (10 men and 1 woman). The pathogen was not identified at that time, but there was a strong suspicion that it was a virus. The patients reported came from similar geographical areas – mountain areas (Tranylvania region - Abrud, Miercurea Ciuc, Odorheiul Secuiesc), which could be a reservoir of infection. Of all, 2 patients died in the first hours after admission, 9 patients gradually recovered their renal function, 4 patients required hemodialysis and 4 patients fully recovered renal function at 1 year. The symptoms were characterized by fever, chills, headache, intense muscle pains, hemorrhagic syndrome (gum bleeding, epistaxis, hematemesis, melena) followed by gastrointestinal symptoms and renal symptoms (non-selective proteinuria of 2 to 15 g/day and hematuria). Histological informations were obtained from the autopsies of the two fatal cases and from the renal biopsies performed in seven of the surviving patients. The autopsies showed intense hemorrhages spreading over the pyramids and light microscopy revealed intense congestion in the renal pyramids and prominent hemorrhages within the tubules and interstitium [7].
In 2008 was diagnosed the first case of hemorrhagic fever with renal syndrome in Romania by Nephrology team of the “Dr. C. I. Parhon” Hospital in Iasi; this case was serologically confirmed. Five more cases were detected until 2012. Five patients were living at the countryside and the sixth one had been on a night trip in the mountains. All six patients presented with fever, hemorrhage, abdominal pain, vomiting +/- diarrhea, and oliguria. All patients survived and five of them completely recovered their renal function, while only one patient retained a mild impairment of the glomerular filtration rate. The treatment consisted of general supportive care and hemodialysis for five patients. What is interesting to note is the high percentage of patients with proteinuria over 3 g / 24h, respectively 7 patients out of 9 (78%). So, the presence of a nephrotic proteinuria is characteristic of nephritis associated with Hantavirus infection . All six patients had both IgM- and IgG-type anti-Hantavirus antibodies, as detected by ELISA assay. Immunoblotting assay detected Dobrava virus and Hantaan virus serotypes [8].
Hantavirus infection has to be suspected everytime a patient presents with fever, abdominal pain, AKI and thrombocytopenia; diagnosis is confirmed based on exposure history and/or endemic areas, clinical manifestations, serology detection of antibodies (by indirect ELISA for IgM and IgG; IFA), kidney biopsy (where interstitial hemorrhage is the main characteristic), detecting viral antigens in tissues by immunohistochemistry or detection of viral RNA in blood or biopsy material[3]
An important way to confirm hantavirus infection is by renal biopsy. The histological findings in hantavirus nephritis are peritubular capilaritis without important tubulitis and hemorrhage is limited in the medulla [5]. Interstitial oedema and inflammatory cell infiltrates (lymphocytes, monocytes, macrophages and polymorphonuclear leucocytes) are usually seen. In electron microscopy, podocytes foot process effacement was described in some studies with hantavirus infection [9,10]
In the case of our patient, simultaneously with the renal biopsy, anti-leptospira antibodies and anti-hantavirus antibodies were determined. Anti-leptospira antibodies were negative. Anti-hantavirus IgM antibodies were detected for the Hantaan serotype. As a conclusion, the serological results correspond to the histological results.
Hantavirus infection can be quite difficult to diagnose because the symptoms are nonspecific and the disease could be unfamiliar to physicians. So, the differential diagnosis should be made with other acute infectious and non-infectious diseases such as: leptospirosis (where you can also have fever, thrombocytopenia, acute kidney injury and acute hepatitis; severe jaundice do occur in both diseases, but is typical for Leptospira and rare for Hantavirus; diagnosis should be excluded by serology tests), hemolytic uremic syndrome (hemolytic anemia and schizocytes in the peripheral blood can help the differential diagnosis), acute surgical abdomen (the intense abdominal pain associated with fever, vomiting, leukocytosis can mimic acute surgical abdomen and a computer tomography exam can be made for additional informations, or even an exploratory laparotomy [8].
Balkan endemic nephropathy (BEN) is a familial, chronic kidney disease with slow and progressive loss of kidney function, that affects the Balkan countries only known as endemic areas: Bulgaria, Romania (Banat and Oltenia regions), Croatia, Serbia, Bosnia. For a person to have the disease, he must live in the endemic area for at least 15-20 years, that’s why the disease has never been diagnosed in children. BEN is associated with a high risk of developing upper urinary tract tumors [11]. The etiology of BEN includes genetic factors and environmental agents such as: heavy metals (lead, cadmium, selenium), polycyclic aromatic hydrocarbons, fungal toxins (Ochratoxin A), aristolochic acids (AAs), viruses (Coronavirus, Hantavirus) [12]. The first cases of BEN were described in Bulgaria in 1956 and in Romania in 1961. The symptoms are non-specific; the first period is asymptomatic, followed by weakness, lumbar pain, pallor and after a long period of time, anemia, proteinuria and chronic kidney disease can be seen. Histological findings are characterized by interstitial fibrosis, tubular atrophy, glomerular fibrosis, arteriolar hyalinosis. There are no pathognomonic signs in BEN, but the set of epidemiological, clinical and histological data is very suggestive for this entity [13].
The only measures to minimize the risk of infection with hantavirus are public awareness and precautionary measures such as cleaning up around houses and barns, eliminating rodent’s shelters and reducing food sources, avoiding contact with potentially contamined areas [14].
Hantavirus infection is a self-limiting infection, it has a transient evolution with an average of about 2-3 weeks, so the treatment si not a targeted one, but only a supportive treatment including fluid, acid-base balance correction, analgesic and antipyretic treatment. Patients have a very good prognosis both in the short and long terms and hemodialysis is required occasionally (in < 5% of the cases) [15]. In cases of severe thrombocytopenia with a high bleeding risk, platelet transfusions may be needed.
The efficacy of Ribavirin therapy in reducing mortality in patients with HFSR was first studied in China. Studies have shown that Ribavirin given in the first five days after the onset of the symptoms decreases the mortality rate [16-17].
Recent studies showed that early administration of intravenous Ribavirin reduces the severity of AKI and the occurrence of oliguria [18].
The only way of really minimizing the risk of hantavirus infection si to get vaccinated. At this moment, there is no vaccine approved for widespread use in Europe or USA. On the other hand, in Asia, respectively in China and Korea, several types of vaccines are used to prevent hantavirus infection (formalin-inactivated vaccines from animal tissues; vaccines derived from formalin-inactivated HTNV-infected suckling mouse brain) [19-21].
A study from Chile that enrolled 60 patients with cardio-pulmonary syndrome related to Andes virus (ANDV) infection analyzed the results of corticosteroids treatment. Half of the patients received placebo and the other half received high doses of methylprednisolone. No significant differences in mortality were observed between treatment groups. Moreover, methylprednisolone did not provide safety and efficacy, although it would have seemed beneficial given the pathogenic mechanisms underlying hantavirus infection (the endothelial cell damage, the cytokine storm with increased capillary permeability) [22-25].
All the data and information obtained in our case study are identified and correlates with the data from the literature:
Author Contributions: Conceptualization, A.C and G.I.; additional analyses, AZ., P.C.G., D.C.B., and G.N.; writing—original draft preparation, S.M.A., J.L.J.H. and J.S.; writing—review and editing, A.Z, A.C.; supervision, G.I.; project administration, PCG. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Written informed consent has been obtained from the patient to publish this paper.
Conflicts of Interest: The authors declare no conflict of interest.