- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 6: e1. DOI 10.35630/2022/12/6.14
One of the main external factors that contributes to the seasonal functional reorganization of the organism in the process of adaptation is seasonal photoperiodic variability. Day length is affecting not only visual function, but also all physiological and behavioural processes controlled by the circadian rhythm system. The person's ability to adapt to changes in external factors is especially important for medical students who experience significant mental overload in the process of studying at a university.
The study goal was to investigate the features of the main hemodynamic parameters and heart rate variability in medical students in the spring and autumn periods, with taking into account gender differences.
Materials and methods: A cross-sectional study of 304 students of the North-Ossetian State Medical Academy (Russia) comprising 233 female and 71 male subjects was performed during periods of elongation (spring) and shortening (autumn) of the photoperiod. Blood pressure (BP), pulse rate (HR), parameters of heart rate variability (HRV) were measured.
Results: The students showed an increase in DBP and MBP rates within the normal range in autumn. Hemodynamic parameters of SBP, DBP, PBP, MBP in males were significantly higher than in females group, regardless of the season. Interseasonal differences in HRV parameters (LF, THF, PHF, PLF, LF/HF, IC) were revealed. From spring to autumn period, LF decreases from 980.22 (590.30; 1445.83) to 690.68 (438.81; 1170.15) ms2 against the background of a decrease in the dominant period of the high-frequency component of the spectrum (THF) from 5.40 (3 .77; 6.10) to 3.71 (3.08; 5.54) s. The contribution of the high-frequency component (PHF) to the total power of HRV fluctuations increases by 10.4%, and the low-frequency component (PLF) decreases by 6.45% as the photoperiod shortens. The centralization index (IC) also declines from 2.58 (1.42; 3.90) to 1.61 (0.97; 2.98); p=0.0076.
Parameters of Mean, Mo, RMSSD, pNN50, SDNN, TP, HF, PHF in spring are lower in males in comparison with females, while parameters of HR, SI, PLF, LF/HF, VLF/HF, IC, PARS are higher in males.
Conclusions: It was indicated that the change in the photoperiod trend is an important mediator of heart rate variability in the annual cycle. The lengthening of the photoperiod in the spring season is accompanied by a shift in the vegetative balance of students towards an increase in the tonus of the sympathetic division of the autonomic nervous system (ANS) together with the involvement of the central circuit of the heart rhythm regulation. In autumn, on the background of shorter daylight duration hours, parasympathetic influences are intensified and autonomic regulation circuit is most active. In the common trend of increased sympathetic activity of the ANS and activation of the central regulatory circuit in the spring, in young males these changes are indicated at greater extent.
Keywords: photoperiod, heart rate variability, cardiovascular system, seasons, students.
The environment and human health are inseparably linked, and that requires in-depth study of the interaction between exogenous environmental factors with the endogenous environment of the body. Students are related to a special social group of young people who undergo significant mental stress in the conditions of increasing negative trends in modern society in the form of depression, anxiety, aggression and require adaptation to new living conditions [1, 2, 3]. The most informative criteria of the adaptation process are indicators of the functioning of the cardiovascular system (CVS). The rhythm of heart contractions reacts to stress-factors, and therefore, the study of heart rate variability is one of the sensitive and informative methods for assessing the state of the human body [4, 5]. The autonomic nervous system (ANS) is the first to react in situations requiring the involvement of adaptive mechanisms and psychofunctional tension in response to the influence of various factors, while the CVS acts as an indicator of human body reactions. Seasonal photoperiodic variability is one of these factors, as the factor of lighting contributes to seasonal functional restructuring [6]. The photoperiod is a predictive factor for the chronoperiodic system of the organism [7, 8]. It regulates the seasonal physiology in many mammals. Photoperiodic information is decoded by the main circadian clock in the suprachiasmatic nucleus (SCN) of the hypothalamus, transmitted through the secretion of melatonin by the pineal gland, and then this neurochemical signal is interpreted by pars tuberalis (PT), expressing melatonin receptors, to control physiological processes [9]. Changing in the lighting mode causes an entire restructuring of the chronoalgorithm in the human body. The available data on seasonal differences in the functional state of the body are rather contradictory and ambiguous, they are varying depending on the climatic and geographical characteristics of the research region [10, 11, 12]. In this regard, the research of the functional state of CVS system of medical students in transitional photoperiods when adapting to an increase and decrease in daylight hours is relevant and important.
The study goal was to investigate the hemodynamic parameters and heart rate variability in medical students during spring and autumn seasons, taking into consideration gender differences.
Three hundred and four 2- and 3-year students at North-Ossetian State Medical Academy (NOSMA) were participating in the clinical observational study. The study was approved by the Ethics Committee of the Institute of Biomedical Research of the Vladikavkaz Scientific Center of the Russian Academy of Sciences (Protocol No. 7 of February 20, 2019). The study is registered at ClinicalTrials.gov (NCT04851080). Participants were informed on the terms of the study and signed the informed consent. Healthy individuals were eligible and subjects with cardiovascular disease were excluded from the study. 233 females (mean age 20.2 ± 1.32 years) and 71 males (mean age 20.5 ± 1.47 years) were evaluated during spring and autumn transitional periods. Blood pressure (BP), morning resting pulse rate (HR) and heart rate variability (HRV) were measured. BP was measured twice with a 2-minute interval by the auscultatory method. When BP measurements differed by > 5 mmHg, BP was remeasured after 2 minutes, and the arithmetic mean of the last two measurements was calculated. HR was measured using a CMS 50DL pulse oximeter. The following parameters were estimated according to well-known formulas, based on available data: pulse pressure (PP, mm Hg), mean blood pressure (MBP, mm Hg). To assess the activity of the autonomic nervous system, the Kérdö index (KI) was calculated as follows: KI = 100 × (1-DBP/HR).
HRV was studied using the hardware-software complex "Varicard 2.51". The examination was carried out 1.5–2 hours after eating in a separate room with a comfortable temperature (20–22°C) and absence of external distraction factors. Recording was done for 5 minutes (Short-term Recordings) with preliminary rest for 5-7 minutes in sitting position in conditions of silence and dimed lighting. The sequence of RR intervals was subjected to automatic and visual analysis for the presence of artifacts and arrhythmias, followed by their consecutive exclusion from the analysis. In accordance with the standards of the European Society of Cardiology and the North American Society of Electrophysiology, we studied two groups of HRV parameters: time parameters (Time Domain Methods) and frequency parameters (Frequency Domain Methods) [13]. Were analysed the following main time indicators of HRV: heart rate (HR, beats/min); average duration of RR intervals (Mean, ms); duration mode of RR intervals (Mo, ms); the square root of the sum of the differences of a consecutive raw of cardiointervals (RMSSD, ms); standard deviation of the array of RR intervals (SDNN, ms); percentage of pairs of cardio intervals with difference of more than 50 ms from the total number in the full array (pNN50, %); the difference between the maximum and minimum values of the duration of RR intervals (MxDMn, ms). All difference values reflect the activity of the parasympathetic division of the autonomic nervous system and belong to the autonomous control circuit to some extent. Stress index (SI), which reflects the degree of tension in regulatory systems and the predominance of the activity of central regulatory mechanisms over autonomous ones was analyzed as well. Further were analyzed following frequency indicators: the total power of the HRV spectrum (TP, ms2); power of the high-frequency spectrum (0.4–0.15 Hz) component of the spectrum (HF, ms2); low-frequency spectrum power (0.15–0.04 Hz) component of the spectrum (LF, ms2); power of the spectrum of the ultra-low-frequency (0.04-0.003 Hz) component of the spectrum (VLF, ms2); the ratio of values (LF/HF, VL/HF), characterizing the ratio of activity levels between the central and autonomous regulation networks; dominant periods of high-frequency (THF, s), low-frequency (TLF, s) and extra-low-frequency (TVLF, s) components of the spectrum; centralization index (IC), evaluating the degree of centralization for heart rate control; spectrum power of high-frequency (PHF, %), low-frequency (PLF, %), super-low-frequency (PVLF, %) components of variability in percentage of the total power of the HRV spectrum, characterizing the levels of activity of various chains of regulation. For an integral quantitative assessment of the functional state of the human body we analyzed indicator of the activity of regulatory systems (IARS, scores), calculated by a special algorithm of R.M. Baevsky and characterizing functional reserves from a point of view of adaptability to environmental conditions [4]. The HRV parameters obtained were compared with the Out_Wind software indicators generated by program in the form of a table, where normative ranges of typicality assessments are formed automatically depending on the patient's age. Because age range of the student test group was similar, the normative ranges of estimates of the typicality of the parameters were applicable to the entire focus group. Statistical analysis of the obtained data was carried out using the computer application Statistica 10.0 software. The characteristics of the studied parameters are presented as mean sample values (M), standard deviation (SD), confidence intervals (±CI) for data with a normal distribution, as well as median sample data (Me), first (Q1, 25%) and third (Q3, 75%) quartiles in the alternative case. The probability of intergroup differences in parameters with a normal distribution was assessed by Student's test; with a distribution other than normal - according to the Wilcoxon test in dependent groups; Mann-Whitney test was applied for the assessment in independent groups. The critical significance level was ≤0.05.
Analysis of the main parameters of hemodynamics showed that the studied indicators were within the normal range according to the classification of WHO, in both increasing and decreasing daylight hours (Table 1). In the autumn season, a slight increase in DBP and MBP in comparison with spring season was noted in student group.
Table 1. Main hemodynamic parameters, Kérdö index of medical students, statistical significance of its differences
| Parameters | Spring | Autumn | р |
||||
| M±SD | CI | M±SD | CI | ||||
| -95,0% | +95,0% | -95,0% | +95,0% | ||||
| HR | 85,9±11,96 | 83,57 | 87,26 | 84,6±10,68 | 80,2 | 84,9 | 0,0789 |
| SBP | 106,0±11,58 | 104,22 | 107,73 | 108,7±11,63 | 106,1 | 111,3 | 0,0998 |
| DBP | 66,3±9,66 | 64,84 | 67,78 | 70,9±8,33 | 69,1 | 72,8 | 0,0003 |
| PP | 39,7±9,58 | 38,21 | 41,12 | 37,8±9,40 | 35,7 | 39,9 | 0,1629 |
| MBP | 79,53±9,30 | 78,12 | 80,94 | 83,5±8,46 | 81,6 | 85,4 | 0,0017 |
| KI | 20,6±16,72 | 18,05 | 23,16 | 16,0±13,17 | 13,12 | 18,99 | 0,0338 |
The obtained KI values indicate the presence of sympathicotonia in students during the studied periods, with a significant increase of the index in the spring season. As a result of the gender analysis of the main hemodynamic parameters, it was concluded that the parameters of heart rate, SBP, DBP, PP, MBP in the spring season are higher in males than in females. This ratio is also preserved in the autumn season (Table 2).
Table 2. Main hemodynamic parameters, Kérdö index of males and females, statistical significance of its differences
| Parameters | Spring | р |
Autumn | р | ||
| Females | Males | Females | Males | |||
| М±SD | М±SD | М±SD | М±SD | |||
| HR | 83,5±11,93 | 92,3±10,90 | 0,0000 | 82,1±10,79 | 86,6±10,41 | 0,4492 |
| SBP | 102,7±9,98 | 118,2±7,28 | 0,0000 | 106,2±10,91 | 116,4±10,63 | 0,0006 |
| DBP | 64,1±8,56 | 74,7±8,16 | 0,0000 | 69,8±7,95 | 74,3±8,76 | 0,0408 |
| PP | 38,7±9,39 | 42,9±9,31 | 0,0143 | 36,4±8,64 | 42,1±10,60 | 0,0209 |
| MBP | 78,0±8,94 | 85,5±7,22 | 0,0000 | 81,9±8,08 | 88,3±7,99 | 0,0036 |
| KI | 21,3±17,17 | 17,9±14,90 | 0,2924 | 16,6±13,45 | 14,8±12,41 | 0,4806 |
As noted in a cohort study, persons over 18 years of age, had shown a seasonal dynamics of HR with a steady decrease to minimum values at the end of summer season [14]. An intragroup interseasonal analysis of hemodynamic parameters showed that females had higher SBP (p=0.0040), DBP (p=0.0000), MBP (p=0.0023) in autumn than in spring season. In males, SBP (p=0.0300) and HR (p=0.0300) are lower in autumn than in spring season. This indicates an increase in the chronotropic reserve of the heart in males in autumn season on the background of a shortening of the photoperiod, which was also noted in their peers in the northern region [15]. KI values in spring season demonstrates an increase in the sympathetic influences of the ANS with an increasing photoperiod, in both males (17.9±14.9 vs. 14.8±12.41) and females (21.3±17.17 vs. 16.6±13 .45); (p=0.0523). It is known that intensity and duration of the visible sun radiation is depending on the season, having signal character and reflexively determines daily biorhythm of human activity through the organs of vision, moreover, in the red spectrum it gives a thermal effect, and in the violet spectrum - a photochemical one [16] . Under the conditions of our research, spring and autumn are comparable by length of the day (7.3 versus 7.2 hours), but differ in the dynamics of changes in illuminance. During spring season, physiological processes are activated because of an increase in the duration of daylight hours, and at the same time instability of the CVS functions may be noted. At the same time, studying at a medical university requires from students to be highly resistant to extreme stress, the ability to adapt to new conditions of existence, and the process of adaptation itself requires tension in the all body's regulatory systems. HRV reflects the result of the dynamic interaction of these systems, hierarchical and non-linear in essence, with the external environment, and relation of the activity of parasympathetic and sympathetic influences can act as a physiological criterion for prediction of various states of the human body [2].
Table 3 represents the main indicators of heart rate variability in spring and autumn season for the entire group. Were revealed interseasonal differences in the parameters of LF, THF, PHF, PLF, LF/HF, IC. On the background of a decrease in the power of the low-frequency component of the spectrum (LF), the dominant period of the high-frequency component of the spectrum (THF) decreases from spring to autumn season as well, that characterizes the weakening of sympathetic influences together with reduction the day length.
Table 3. HRV parameters of students and statistical significance of their differences in the spring/autumn periods
Parameters |
Spring | Autumn | р |
| Ме (Q1; Q3) | Ме (Q1; Q3) | ||
| HR, уд./мин. | 84,78 (76,41; 93,57) | 85,14 (78,45; 92,37) | 0,9262 |
| Mean, мс | 707,70 (641,25; 785,21) | 704,70 (649,55; 764,80) | 0,9262 |
| RMSSD, мс | 33,80 (24,06; 51,79) | 34,33 (25,07; 54,48) | 0,7981 |
| pNN50, % | 9,47 (4,23; 22,20) | 11,71 (4,58; 23,97) | 0,4768 |
| SDNN, мс | 53,16 (42,20; 66,76) | 51,53 (37,75; 66,72) | 0,3569 |
| Mo, мс | 688,00 (629,50; 772,50) | 680,0 (636,0; 759,50) | 0,8023 |
| SI | 118,68 (72,68; 214,98) | 130,19 (75,91; 261,16) | 0,3744 |
| TP, мс2 | 2346,74 (1535,83; 3558,81) | 1852,29 (1149,22; 3449,86) | 0,1297 |
| HF, мс2 | 534,29 (283,75; 1089,57) | 493,59 (295,52; 1134,10) | 0,8696 |
| LF, мс2 | 980,22 (590,30; 1445,83) | 690,68 (438,81; 1170,15) | 0,0060 |
| VLF, мс2 | 344,50 (211,86; 531,67) | 278,39 (162,83; 488,22) | 0,0807 |
| THF, с | 5,40 (3,77; 6,10) | 3,71 (3,08; 5,54) | 0,0000 |
| TLF, с | 10,56 (9,27; 12,96) | 9,89 (8,90; 12,82) | 0,1693 |
| TVLF, с | 46,55 (37,93; 58,56) | 46,55 (36,57; 53,89) | 0,8334 |
| PHF, % | 27,91 (20,42; 41,39) | 38,28 (25,11; 50,76) | 0,0076 |
| PLF, % | 49,47 (40,19; 58,89) | 43,02 (33,12; 53,19) | 0,0025 |
| PVLF, % | 17,93 (13,04; 23,92) | 18,75 (12,00; 24,13) | 0,8538 |
| LF/HF | 1,87 (0,98; 2,82) | 1,14 (0,74; 1,96) | 0,0036 |
| VLF/HF | 0,64 (0,34; 1,10) | 0,51 (0,30; 0,96) | 0,1304 |
| IC | 2,58 (1,42; 3,90) | 1,61 (0,97; 2,98) | 0,0076 |
| IARS | 5,0 (3,0; 6,0) | 4,0 (3,0; 6,0) | 0,1438 |
During analysis of the contribution of waves of different nature into the total capacity of the spectrum of variability, it was found that PHF, which characterizes the relative level of activity of the parasympathetic chain of regulation, shows a positive trend from spring to autumn (Fig. 1). The contribution of this high-frequency component to the total capacity of HRV fluctuations increases by 10.4%, and as result, there is an increase in the parasympathetic effects of the ANS on the heart rhythm. On the contrary, the contribution of the low-frequency component (PLF) into the total capacity of HRV fluctuations decreases by 6.45%, which indicates a decrease in the sympathetic influences of the ANS on CVS activity in the autumn period. Moreover, the negative dynamics of LF/HF from spring to autumn seasons characterizes the change in the ratio of activity levels of the central and autonomous regulatory circuits in favour of the latter. Confirmation of the decline in the level of activity of the central regulation circuit in the autumn is the decrease in the centralization index among students from 2.58 (1.42; 3.90) to 1.61 (0.97; 2.98); p=0.0076.
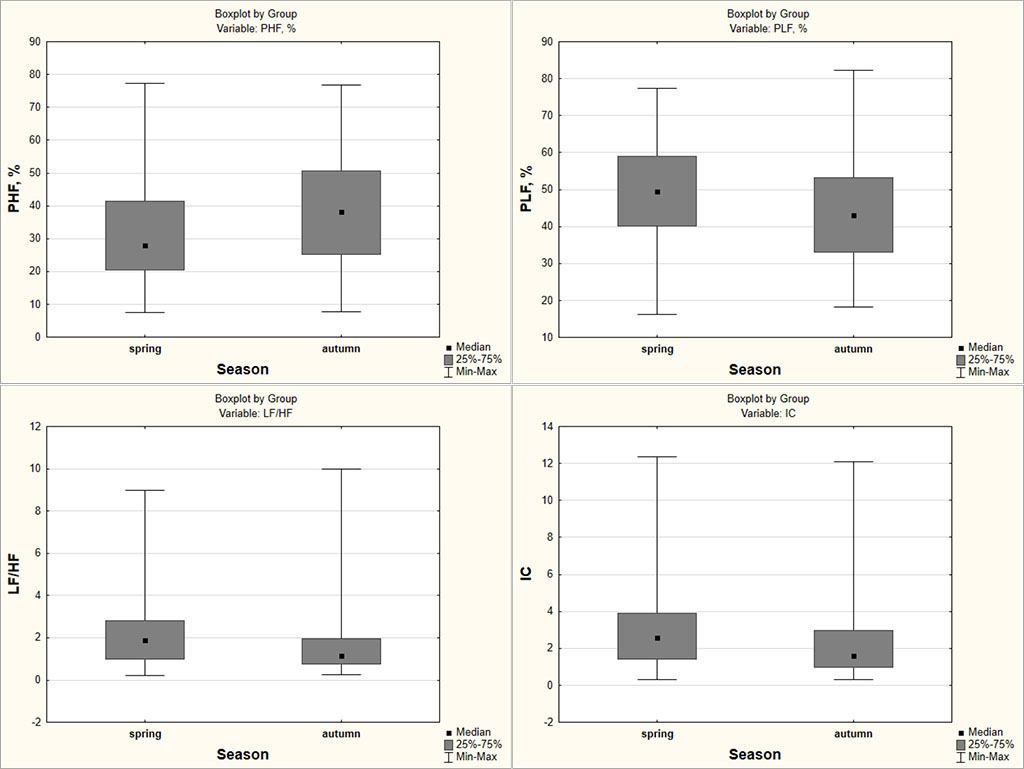
Fig. 1. Dynamics of parameters PHF, PLF, LF/HF, IC among medical students in spring/autumn periods
It is also established that the relative contribution of VLF, which reflects the level of activity of cerebral ergotropic structures, to the total capacity of the HRV spectrum remains relatively stable, and fluctuations in the spectral structure occur due to the high-frequency and low-frequency components of the spectrum, which correspond to the parasympathetic and sympathetic divisions of the ANS. In the spring season, in a comprehensive assessment of HRV according to the integral indicator IARS (5.0 (4.0; 6.0)), which characterizes conditional "price of adaptation", a state of pronounced tension of regulatory systems was identified in students on the background of an increase in the duration of daylight hours. This is associated with mobilization of adaptation mechanisms and increased activity of the sympathetic-adrenal system and the pituitary-adrenal system. At the same time, some authors note that under conditions of everyday lifestyle, the role of photoperiodism can be masked by social factors [17, 18]. The intensification of the learning process has increased in recent years and this factor can lead to a decrease in the adaptive capabilities of students against the background of an increase in the activity of the sympathetic department of the ANS [19], autonomic dysfunction [20]. Under the conditions of the circumpolar region, the functional restructuring of cardiac activity regulation can occur with a simultaneous increase in the tone of both the sympathetic and parasympathetic divisions of the ANS in different seasons of the year [21], which corresponds to the concept of vegetative flexibility, which ensures the body's ability to successful adaptation. Thus, in an experiment with Phodopus sungorus, it was noted that high-frequency heart rate variability, a relatively pure index of vagal-parasympathetic control of the heart, was markedly enhanced by chronic exposure to short day, and pharmacological blockade of the sympathetic and parasympathetic branches led to the coactivation of two sections of the ANS in animals with short day lengths [22]. However, it should be taken into account that in this experiment the photoperiods had no trend and were constant: long day lengths (16:8 light–dark), short day lengths (8:16 light–dark).
In autumn period, the values of IARS (4.0 (3.0; 6.0)) in our study correspond to the state of moderate tension of regulatory systems. As a result of the gender analysis of HRV parameters, it was found that in males the parameters Mean, Mo, RMSSD, pNN50, SDNN, TP, HF, PHF are lower in spring, while HR, SI, PLF, LF/HF, VLF/HF, IC, PARS are higher than in females in the same season (Table 4).
Table 4. HRV parameters of females and males, statistical significance of their differences in the spring/autumn periods
Parameters |
Spring | Autumn | ||||
| Females | Males | р | Females | Males | р | |
| Ме (Q1; Q3) | Ме (Q1; Q3) | Ме (Q1; Q3) | Ме (Q1; Q3) | |||
| HR, r/min | 83,47 (74,38; 90,88) | 92,13 (83,97; 100,92) | 0,0001 | 85,15 (78,33; 91,63) | 85,14 (79,77; 97,20) | 0,4204 |
| Mean, ms | 718,80 (660,22; 806,71) | 651,25 (594,55; 714,56) | 0,0001 | 704,65 (654,78; 765,97) | 704,75(617,25; 752,16) | 0,4204 |
| RMSSD, ms | 36,28 (26,83; 53,24) | 24,83 (19,14; 34,86) | 0,0005 | 34,59 (25,93; 56,17) | 30,10(19,15; 45,10) | 0,1793 |
| pNN50, % | 12,14 (4,70; 24,86) | 4,60 (2,25; 9,73) | 0,0012 | 11,74 (5,23; 28,88) | 8,49(1,65; 19,39) | 0,2268 |
| SDNN, мс | 55,91 (43,40; 68,94) | 48,43 (38,31; 59,91) | 0,0417 | 52,87 (39,86; 66,72) | 45,94(33,47; 64,35) | 0,2873 |
| Mo, ms | 702,00 (639,00; 789,00) | 636,00 (578,00; 698,00) | 0,0001 | 682,00 (644,00; 760,00) | 672,0(601,0; 729,00) | 0,3472 |
| SI | 110,78 (67,69; 194,07) | 185,99 (105,53; 261,42) | 0,0115 | 129,46 (66,95; 222,27) | 163,47(90,0; 329,15) | 0,1931 |
| TP, ms 2 | 2477,41 (1574,36; 3771,43) | 1786,39 (1271,26; 2648,45) | 0,0429 | 1898,66 (1182,10; 3463,26) | 1640,80(949,82; 2976,74) | 0,5629 |
| HF, ms 2 | 630,38 (320,90; 1232,38) | 361,26 (205,75; 554,99) | 0,0028 | 539,95 (345,94; 1312,56) | 437,23(195,89; 892,28) | 0,1861 |
| LF, ms 2 | 1077,33 (583,33; 1449,67) | 896,49 (597,27; 1403,49) | 0,5479 | 700,27 (455,97; 1185,76) | 614,60(435,71; 1091,95) | 0,6947 |
| VLF, ms 2 | 366,16 (217,35; 556,34) | 293,73 (168,15; 423,13) | 0,1918 | 280,40 (192,83; 447,48) | 214,17(138,41; 673,12) | 0,6273 |
| THF, s | 5,28 (3,57; 5,99) | 5,89 (4,97; 6,36) | 0,0032 | 3,34 (3,00; 4,55) | 5,00(3,58; 5,66) | 0,0198 |
| TLF, s | 10,78 (9,31; 13,13) | 10,34 (8,75; 12,64) | 0,6066 | 10,24 (9,06; 13,47) | 9,48(8,33; 10,67) | 0,1495 |
| TVLF, s | 48,76 (39,38; 60,24) | 39,38 (32,00; 56,89) | 0,1526 | 46,55 (39,38; 53,89) | 44,52(35,31; 56,89) | 0,6488 |
| PHF, % | 31,56 (21,19; 42,23) | 22,17 (17,68; 27,58) | 0,0015 | 39,15 (26,76; 50,27) | 29,31(17,87; 51,24) | 0,1071 |
| PLF, % | 48,59 (36,87; 57,72) | 53,77 (48,54; 62,48) | 0,0026 | 42,08 (33,16; 49,15) | 52,26(28,12; 61,44) | 0,1663 |
| PVLF, % | 17,92 (13,09; 23,51) | 18,94 (13,00; 26,64) | 0,3955 | 18,59 (11,86; 24,52) | 18,92(12,54; 23,60) | 0,7177 |
| LF/HF | 1,47 (0,88; 2,53) | 2,61 (1,94; 3,22) | 0,0008 | 1,06 (0,76; 1,85) | 1,79(0,47; 3,29) | 0,1570 |
| VLF/HF | 0,57 (0,33; 1,00) | 0,87 (0,46; 1,51) | 0,0252 | 0,46 (0,27; 0,94) | 0,57(0,44; 1,65) | 0,1452 |
| IC | 2,17 (1,37; 3,72) | 3,51 (2,63; 4,66) | 0,0015 | 1,55 (0,99; 2,74) | 2,41(0,95; 4,60) | 0,1071 |
| IARS | 4,00 (3,00; 6,00) | 6,00 (5,00; 7,00) | 0,0003 | 4,00 (3,00; 5,00) | 5,00(4,00; 6,00) | 0,1438 |
The obtained data indicates that in spring season, the sympathetic chain of regulation activated to a greater extent in males than in females against the background of pronounced centralization of heart rhythm control. With an increasing photoperiod, IC in males is 38.2% higher than in females and three times higher than the upper limit of normal (0.9-1.3 c.u.); SI is higher by 40.4% than in females and by 36 c.u. exceeds the upper limit of normal (70-150 c.u.). One of possible increase reasons in these parameters in the spring season may be a violation of the sleep/wake cycle. As it was noted in the study [23], in spring season the quality of the sleep/wake cycle worsens in comparison with autumn season, since circadian system of human more easily adapts to delay of phase (autumn) than to advance of phase (spring).
In autumn season, gender differences were revealed only for the dominant period of the high-frequency component (THF), which is significantly lower in females due to an increase in the contribution of the parasympathetic chain to regulatory processes. It became obvious that with a general negative trend of strengthening of the sympathetic and decreasing parasympathetic activity of ANS in the spring season, in males these changes are more pronounced than in females and accompanied by activation of the central regulatory circuit. Increased sympathetic activity in the regulation of heart rate in young males compared to females was also shown in studies [24, 25]. Intragroup analysis revealed positive dynamics of PHF (p=0.0171) in girls against the background of negative dynamics of parameters LF (p=0.0119), THF (p=0.0001), PLF (p=0.0114), LF/HF (p=0.0142), IC (p=0.0171) from spring to autumn, which indicates an increase in parasympathetic influences and a decrease in the degree of centralization of heart rhythm control. In males, significant interseasonal differences were found only for the dominant period of the high-frequency component of the THF spectrum, which decreases from spring to autumn seasons from 5.89 (4.97; 6.36) to 5.00 (3.58; 5.66), (p=0.0258). The observed weakening of sympathetic and strengthening of parasympathetic influences on the heart rhythm of medical students against the background of a hierarchical decrease in the level of regulation from central to autonomous as daylight hours shorten in autumn season corresponds to the classical model of central reciprocal regulatory control of the ANS.
Our study suggests that changing in the trend of the photoperiod is an important mediator of heart rate variability in annual cycle. Changes in photoperiod in opposite seasons (spring/autumn) are accompanied by fluctuations in the levels of vagosympathetic balance of students' ANS. Against the background of an increase in the duration of daylight hours in the spring season, the vegetative balance in students shifts towards an increase in the tone of the sympathetic division of the ANS with the involvement of the central circuit of heart rhythm regulation, while in autumn the autonomous circuit of regulation is most active, parasympathetic influences are increasing as well. Kérdö index values are confirming the strengthening of the sympathetic influences of the ANS with an increasing photoperiod in the spring season, both in males and females. Hemodynamic parameters SBP, DBP, PBP, MBP in males are significantly higher than females, in both spring and autumn seasons.
In the general strengthening the sympathetic and decreasing of parasympathetic activity of the ANS of students in the spring season, in males these changes are more pronounced than in females and accompanied by activation of the central regulatory circuit. A comprehensive evaluation of the functional state of medical students corresponds to the state of moderate tension of regulatory systems in the autumn period and pronounced tension of regulatory systems in spring season. Future research will be focused on studying of the effects of photoperiodism on the autonomic regulation of cardiac activity in combination with other environmental and social factors.
The author declares the absence of obvious and potential conflicts of interest which must be reported in relation to the publication of this article.