- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 5. DOI 10.35630/2024/14/5.506
Microcarcinoma is a thyroid gland cancer with a size of less than 1 cm.
The aim: To determine the frequency of microcarcinoma among malignant pathologies of the thyroid gland based on fine-needle aspiration biopsies (FNAB) data.
Materials and Methods: A retrospective analysis of 2490 FNAB data was conducted under ultrasound control from 2007 to 01.07. 2024 at the Ternopil Regional Clinical Hospital. FNAB results were categorized into four groups: the first group – non-diagnostic results, the second – suspicion of thyroid cancer, the third – thyroid cancer, and the fourth – benign lesions. After the Bethesda system was introduced, FNAB classifications were based on this system.
Results: Thyroid cancer was diagnosed in 186 cases (7.5%). Among these, macrocarcinoma was identified in 152 cases (81.7%) and microcarcinoma in 34 cases (18.3%). The peak incidence of microcarcinoma is observed in the 40-50 age, with approximately 90% of patients being women. Microcarcinomas in solitary nodules were found in 16 cases (47%); among multinodular goiters in 18 cases (53%). In 8 cases (23.5%), microcarcinoma was associated with larger thyroid cancers (multifocal process) and in 4 cases (11.8%) with benign nodules. In 6 cases (17.7%), microcarcinomas were associated with nodules suspicious for cancer.
Conclusions: Indications for FNAB should be guided by the identified sonographic characteristics (solid echostructure of the nodule; hypoechogenicity; vertical orientation of nodules; indistinct margins of the nodule; presence of microcalcifications; regional cervical lymphadenopathy with signs of malignancy).
Keywords: thyroid ultrasound, thyroid nodules, fine needle aspiration biopsy, thyroid microcarcinoma, thyroid cancer.
Thyroid cancer is among the top ten most common cancers worldwide. The ratio of women to men is 10.1/100,000 to 3.1/100,000, respectively [1]. According to the National Institutes of Health (NIH), about 1.3% of the total population will be diagnosed with thyroid gland cancer at some point in their life [2]. A similar situation is observed in Ukraine, where there has been an increase in cases of papillary thyroid cancer over the past 40 years, partly due to the Chernobyl disaster [3]. The increase in thyroid cancer cases is also linked to improvements in diagnostic methods, specifically ultrasound and cytological studies [4].
Fine needle aspiration biopsy is currently considered the "gold standard" for preoperative verification of thyroid cancer with an informativeness of 90-95% [5].
There is no consensus regarding the prognosis of microcarcinoma treatment. Some authors believe that microcarcinomas have a low aggressiveness potential and a favorable prognosis [6,4], while others have reported extrathyroidal spread of the tumor into fat or connective tissue in 24.4% of cases and into muscle in 5.6% of cases [7], as well as metastatic involvement of regional lymph nodes in 22.5-42.6% of cases [7,8]. Shimizu et al. noted multiple metastases of papillary microcarcinoma to paratracheal lymph nodes and lungs [9], and Shawky et al. reported metastasis of papillary microcarcinoma (0.3 cm) to the brain [10].
Thus, the presence of microcarcinoma does not exclude the possibility of extensive surgical and combined treatment.
The aim of the study is to determine the frequency of microcarcinoma among malignant pathologies of the thyroid gland based on fine-needle aspiration biopsies (FNAB) data.
A retrospective analysis of 2490 fine needle aspiration biopsies (FNABs) was performed under ultrasound guidance from 2007 to July 1, 2024 at the surgical department of the Ternopil Regional Clinical Hospital. Ultrasound examination was performed using a 10.0 MHz linear probe. FNABs were conducted under ultrasound guidance employing the "freehand" method according to the traditional algorithm with two projection punctures. In cases of multinodular goiter, possible biopsy sites were punctured. Aspirate samples were stained using the May-Grünwald-Giemsa method. Employing the Bethesda system, FNAB results were classified into 6 categories:
Out of 2490 FNABs, benign processes (Bethesda 2) were detected in 1600 cases, accounting for 64.2%. Suspicion of malignant process (Bethesda 3-5) was found in 527 (21.2%) cases, including 35 cases (6.6%) having a suspicious nodule up to 1 cm, and 24 cases (5%) having a suspicious microfocus nodule combined with lesions over 1 cm. Malignant process was diagnosed in 186 cases, accounting for 7.5%, among them microcarcinomas identified in 34 cases, which represents 1.4% of all biopsies and 18.3% of thyroid cancers, while macrocarcinomas were found in 152 cases, representing 6.1% of all examined patients.
Non-diagnostic results (Bethesda 1) were obtained in 177 FNABs, accounting for 7.1%. The results of fine-needle aspiration are presented in Figure 1.
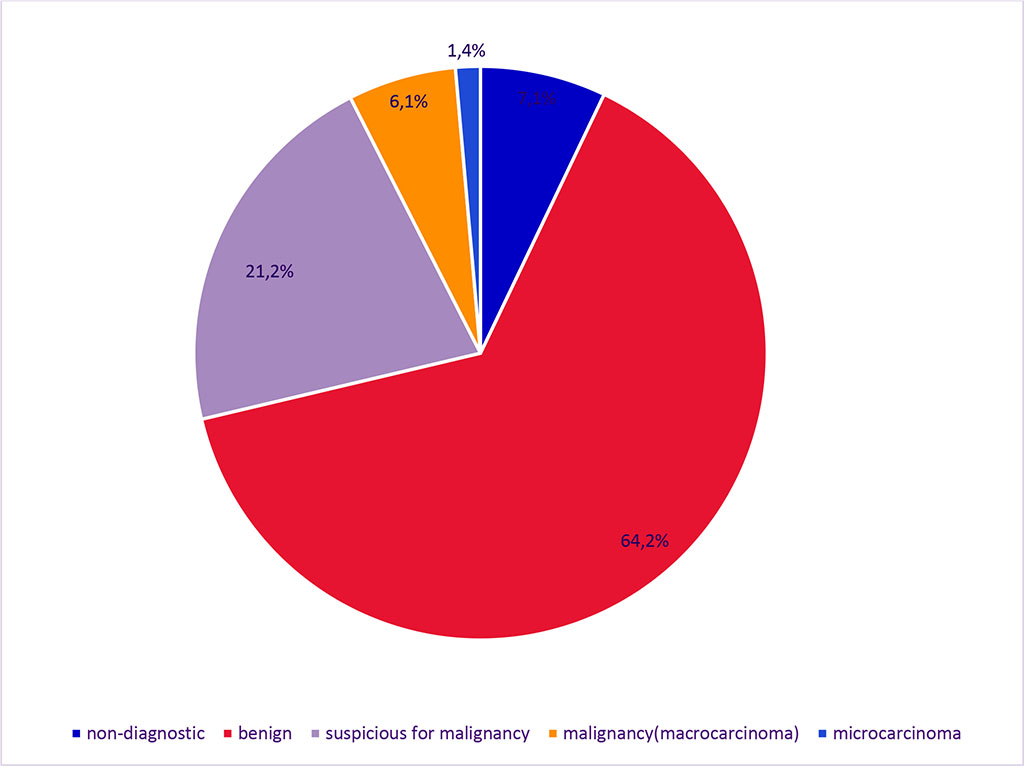
Figure 1. Results of Fine Needle Aspiration Biopsy
Among thyroid gland cancers (186 cases), a macrocarcinoma was identified in 152 cases of FNAB, representing 81.7% of the cases. Papillary thyroid cancer was observed in 135 cases (88.8%); medullary cancer in 12 cases (7.9%), and anaplastic cancer in 5 cases (3.3%) which is presented in figure 2. The highest incidence of macrocarcinoma cases (27%) was observed in individuals over 60 years old. In the 50-59 age group, the incidence was 23.8%, in the 40-49 age group – 19%, in the 30-39 age group – 16.4%; in the 20-29 age group – 11.8%, and only 2% in patients under 20 years old. The ratio of women to men was 5:1, namely 84.2% women and 15.8% men.
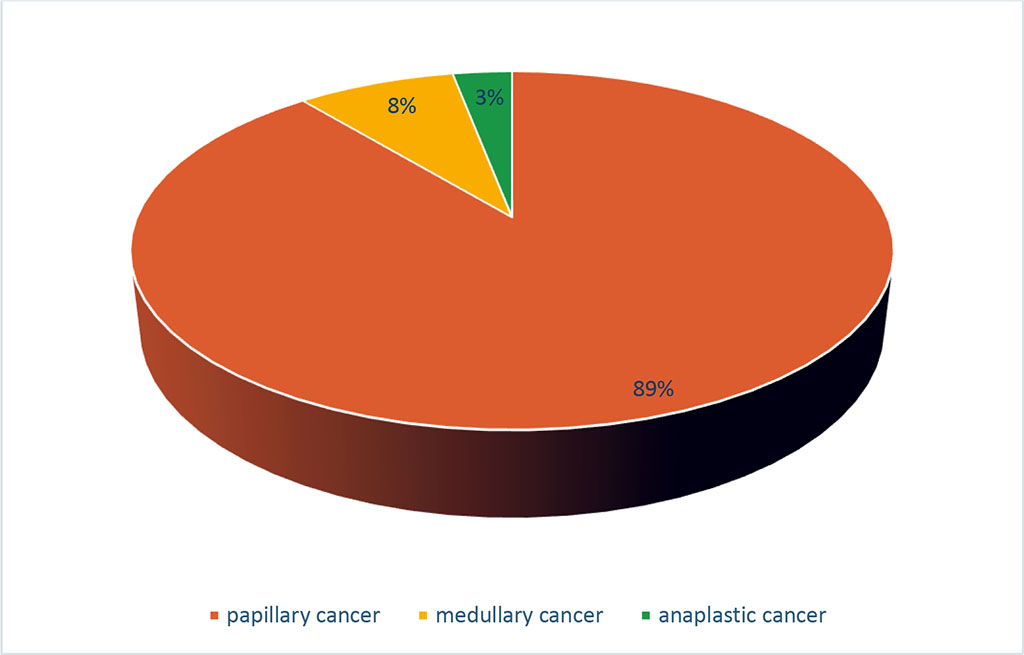
Figure 2. Structure of Macrocarcinoma of the Thyroid Gland by Histological Type
Microcarcinomas were found in 34 cases, representing 18.3% of all malignant thyroid pathology and 1.4% of all punctured patients. The peak incidence of micropapillary carcinoma was observed between 40 and 50 years of age, accounting for 29.4%. Incidence in the 20-29 age group was 11.8%, in the 30-39 age group – 26.5%, in the 50-59 age group – 20.5%, and in individuals over 60 years old – 11.8%, with the majority of patients (94%) being female. Microcarcinomas were found in solitary nodules measuring 6 to 9 mm in 16 (47%) cases, and in 18 (53%) cases, they were found in multinodular goiters. Among these patients, microcarcinomas were associated with thyroid cancer larger than 1 cm in 8 cases (23.5%), and with benign nodules in 4 cases (11.8%). In 6 cases (17.7%), the microcarcinomas were found alongside other nodules suspected of malignancy. The results are presented in Figure 3. All detected microcarcinomas were classified as papillary thyroid cancer.
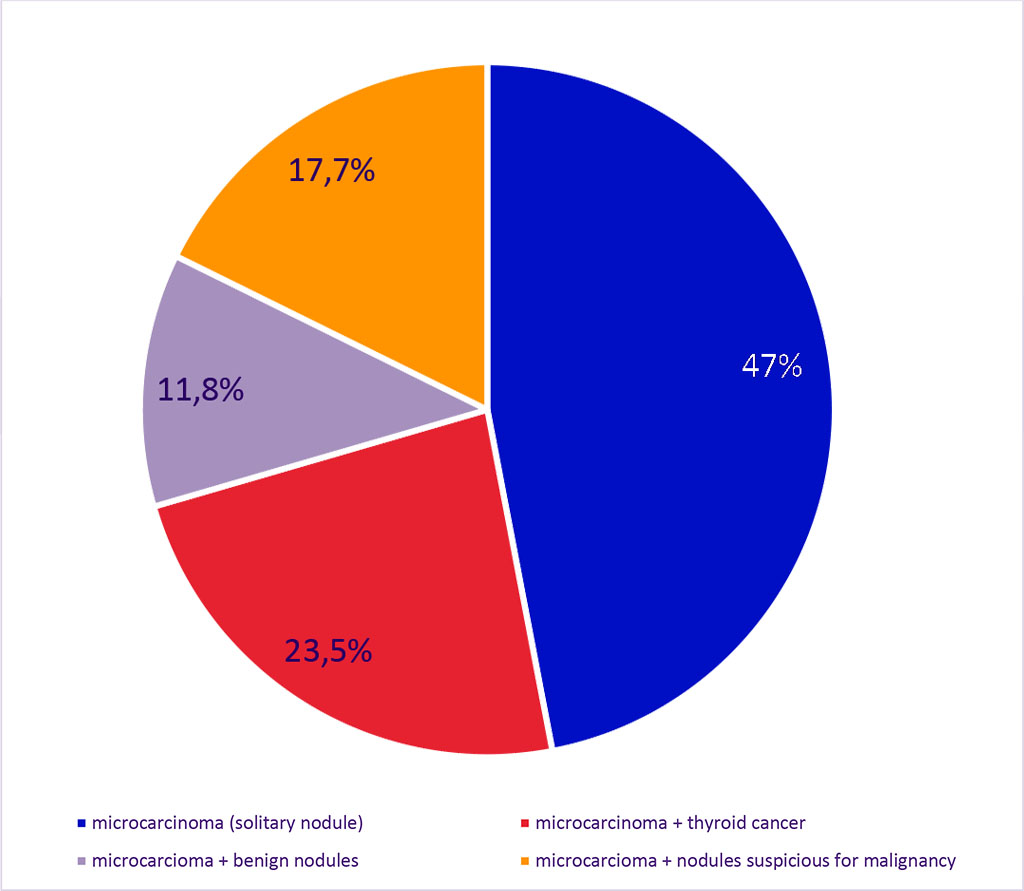
Figure 3. Structure of Micropapillary Carcinoma in Combination with Other Thyroid Nodules
The clinical feature of microcarcinomas is their latent course, which is not accompanied by thyroid dysfunction, nor symptoms of neck organ compression. Palpation allows for the detection of nodules larger than 1 cm in only 8-10% of cases if the nodule is located on the anterior surface of the gland. Thus, palpation-based detection of micropapillary carcinomas is nearly impossible. Additionally, in 80-90% of cases, microcarcinomas are masked by goiter-transformed tissue. Therefore, FNAB under ultrasound guidance remains the primary method for suspecting and diagnosing malignant pathology at the preoperative stage.
Most guidelines, including those from the American Thyroid Association, recommend performing FNAB for nodules larger than 1 cm. For microfocus lesions, it is considered that FNAB should be performed for nodules up to 1 cm if signs of malignancy are present and specific ultrasound features are detected [11]. Since the development of the ACR TI-RADS system, each ultrasound feature is assigned a certain number of points, which collectively determine the malignancy risk and guide treatment recommendations [12].
Table 1. Classification Types of Thyroid Nodular Lesions by Ultrasound (ACR TI-RADS) [12]
| Categories | Signs | Points |
| Composition (Choose 1) | Cystic or almost completely cystic | 0 points |
| Spongiform | 0 points | |
| Mixed cystic and solid | 1 point | |
| Solid or almost completely solid | 2 points | |
| Echogenicity (Choose 1) | Anechoic | 0 points |
| Hyperechoic or isoechoic | 1 point | |
| Hypoechoic | 2 points | |
| Very hypoechoic | 3 points | |
| Shape (Choose 1) | Wider-than-tall | 0 points |
| Taller-than-wide | 3 points | |
| Margin (Choose 1) | Smooth | 0 points |
| Ill-defined | 0 points | |
| Lobulated or irregular | 2 points | |
| Extra-thyroidal extension | 3 points | |
| Echogenic foci (Choose all that apply) | None or large comet-tail artifacts | 0 points |
| Macrocalcifications | 1 point | |
| Peripheral (rim) calcifications | 2 points | |
| Punctate echogenic foci | 3 points |
The management scheme for nodules according to this system is presented in Table 2 [12].
Table 2
| Categories | Management recommendations |
| TR1 (0 points): Benign | No FNA |
| TR2 (2 points): Not suspicious | No FNA |
| TR3 (3 points): Mildly suspicious | FNA if ≥2,5 cm; follow if ≥1,5 cm |
| TR4 (4-6 points): Moderately suspicious | FNA if ≥1,5 cm; follow if ≥1 cm |
| TR5 (7 points): Highly suspicious | FNA if ≥1 см; follow if ≥0,5 см |
However, it is important to note that none of these ultrasound features are absolute indicators of malignancy. Although hypoechogenicity is considered more characteristic of malignant formations, it is not excluded that benign nodules may also have such characteristics. The presence of microcalcifications is characterized by quite high specificity (95.2%), but the sensitivity is 59.3%, and the diagnostic value is 83.8% concerning malignant transformation [13]. In one study, among 64 nodules with peripheral calcifications, 19 (30%) were benign and 45 (70%) were malignant, with interruptions and thickening of calcifications associated with higher malignancy [14]. When calcifications are present, age should also be considered, as the risk of cancer is higher in individuals younger than 40 compared to older patients [13].
Some studies have found that 41.8% of benign small nodules had indistinct margins and 16.5% had irregular shapes [11]. Another study demonstrated that even clear and smooth contours could indicate encapsulated papillary thyroid cancer, observed in 50% of cases. According to these data, in 12-32% of cases, the sonographic image of thyroid cancer does not differ from thyroid adenomas and is characterized by isoecogenicity and a clear "halo" rim around the periphery [7].
The American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE), and Italian Association of Endocrinologists (AME) (2016) recommend considering factors such as head and neck radiation history, family history of medullary thyroid cancer, MEN syndrome or papillary thyroid cancer, age under 14 or over 70 years, male gender, cervical lymphadenopathy, and extrathyroidal nodule growth as indications for FNAB in high-risk nodules measuring 5-10 mm [15].
In our practice, there were three cases of incidental histological detection of micropapillary carcinomas measuring 2, 1, and 2 mm following surgeries for diffuse toxic goiter, multinodular euthyroid goiter with neck organ compression, and multinodular toxic goiter. It is worth noting that in one case (surgery for diffuse toxic goiter), metastases were found in the cervical lymph nodes of groups IV and VI upon further examination.
The authors declare no conflict of interests.