- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 5. DOI 10.35630/2024/14/5.505
The purpose of the study was to determine pathomorphological and pathohistological characteristics of primary vulvar carcinoma.
Materials, methods, and study design: A retro- and prospective comprehensive morphological study of 117 patients was conducted at Bondar Regional Cancer Research Center (Donetsk) over the period from 2002 to 2019. Out of 117 we identified 44 patients with primary vulvar cancer without previous inflammatory diseases (cancer de novo). The materials were fixed in formalin and examined using standard methods of histological staining and morphometry. Statistical analysis methods were used to process the data.
Results and Discussion: The differentiated VIN type occurred in 61.2% of cases, the undifferentiated type – in 37.8%. VIN1 was characterized by basal cell hyperplasia, VIN2 – by abnormal vertical anisomorphy, VIN3 – by significant abnormal cell stratification and differentiation. Cell proliferative activity increased with VIN severity, with the highest mitotic index and nuclear-cytoplasmic ratio in VIN3. In VIN1, a positive reaction to nuclear proliferation antigen was observed in the lower third of the epithelial layer. In VIN2 and VIN3, the positive reaction covered most of the epithelium. Well-differentiated SCC (G1) was characterized by polymorphic cell complexes with a small number of pathological mitoses and apoptosis. Moderately differentiated SCC (G2) had a pronounced inflammatory reaction and necrosis, with disruption of the basement membrane. Poorly differentiated SCC (G3) demonstrated loss of vertical anisomorphy, high mitotic index and presence of tumor emboli in vessels.
Conclusions: The study showed significant differences in the morphological and morphometric characteristics of different types of VIN and invasive vulvar carcinoma. The results will help to improve the diagnosis and treatment of primary vulvar cancer, especially in understanding its pathogenesis and prognostic factors.
Keywords: vulvar cancer, carcinoma, pathomorphology, immunohistochemistry
Vulvar cancer accounts for 3% to 5% of all cases of malignant tumors of the female reproductive system. Due to the increase in life expectancy and high HPV (human papillomavirus) prevalence, there is a tendency for the increasing incidence and mortality from this pathology [1-3]. In modern literature, there are two main versions regarding the pathogenesis of vulvar cancer. It is assumed that one form of vulvar cancer can occur primarily, without previous background inflammatory diseases of the skin and mucous membranes, and such cancer is referred to as "cancer de novo" [4-8].
The purpose of the study: to provide pathomorphological and pathohistological characteristics of primary vulvar carcinoma.
Retro- and prospective complex morphological study included 117 patients with various forms of vulvar cancer were conducted at the G.V. Bondar Regional Cancer Research Center of the Ministry of Health (Donetsk) from 2002 to 2019. Out of 117 patients 44 patients with vulvar cancer, without inflammatory and dystrophic background diseases of the skin and mucous membranes were detected according to clinical and morphological examination (cancer de novo). The description of this group is presented in this article.
The material was fixed in 10% neutral formalin solution. The tissue was embedded in paraffin using standard methods. Serial histological sections 5 μm thick were made on a rotary microtome MPS-2, then stained with van Gieson hematoxylin and eosin stain, Verhoeff stain, toluidine blue at pH 2.6 and 5.3, and a PAS reaction was performed treating control sections with amylase.
The morphometric study was based on the Glagolev’s point-count method. We determined total specific volume of inflammatory infiltrate cells; specific volumes of neutrophils, macrophages, lymphocytes, plasma cells, and labrocytes. In the morphometric studies we followed the principles set out in G.G.Avtandilov's manual [9].
Image analysis was performed on a Hund H500 universal microscope with a television system connected to an OEM IBM PC/AT Pentium personal computer.
Morphological and morphometric studies were conducted in the Department of Pathomorphology of the Central Scientific Research Laboratory at the M. Gorky Donetsk State Medical University.
To obtain mathematical results, we used Statistica 5.5 and MedStat v. 5.2 programs, the D'Agostino-Pearson mathematical criterion for normality testing, descriptive statistics methods, Spearman's rank correlation, comparison of central tendencies of two independent samples, Wilcoxon's test, Student's test, comparison of proportions for two groups, and calculation of the risk ratio. When comparing the distribution of qualitative features, the χ2 criterion was used. In all cases, the difference was considered statistically significant at a level of p <0.05.
The morphology of squamous cell intraepithelial neoplasia in primary vulvar cancer (VIN - according to the classification of the International Society for the Study of Vulvovaginal Diseases (ISSVD)) should include proliferation of atypical basal cells and, accordingly, all 5 criteria of atypia: involvement of the basal layer, enlarged nuclei, hyperchromasia, pleomorphic cells, and an increased number of mitotic figures. In patients with primary vulvar cancer, differentiated VIN type was more frequently detected from histological samples – in 23 (61.2±8.0%) of 37 cases. Undifferentiated VIN was noted in 14 (37.8±8.0%) cases.
The undifferentiated VIN, depending on the level of disturbances in cellular relationships, was graded as VIN1 (mild degree) – 4 cases (10.8±5.1%), VIN2 (moderate degree) – 4 (10.8±5.1%) and VIN3 (severe degree) – 6 (16.2±6.1%).
The differentiated VIN was characterized by absent or weak atypia of the basal or parabasal layers of keratinocytes, preserved PAS-positive basement membrane, positive for type IV collagen in immunohistochemical typing. An increase in the number of prematurely differentiated keratinocytes with abundant eosinophilic cytoplasm and polymorphic nuclei in the deep layers of the epidermis (usually in elongated and branching epidermal outgrowths) was characteristic. Deeply located scaly swirling patterns were revealed, with the formation of keratin pearls (Fig. 1).
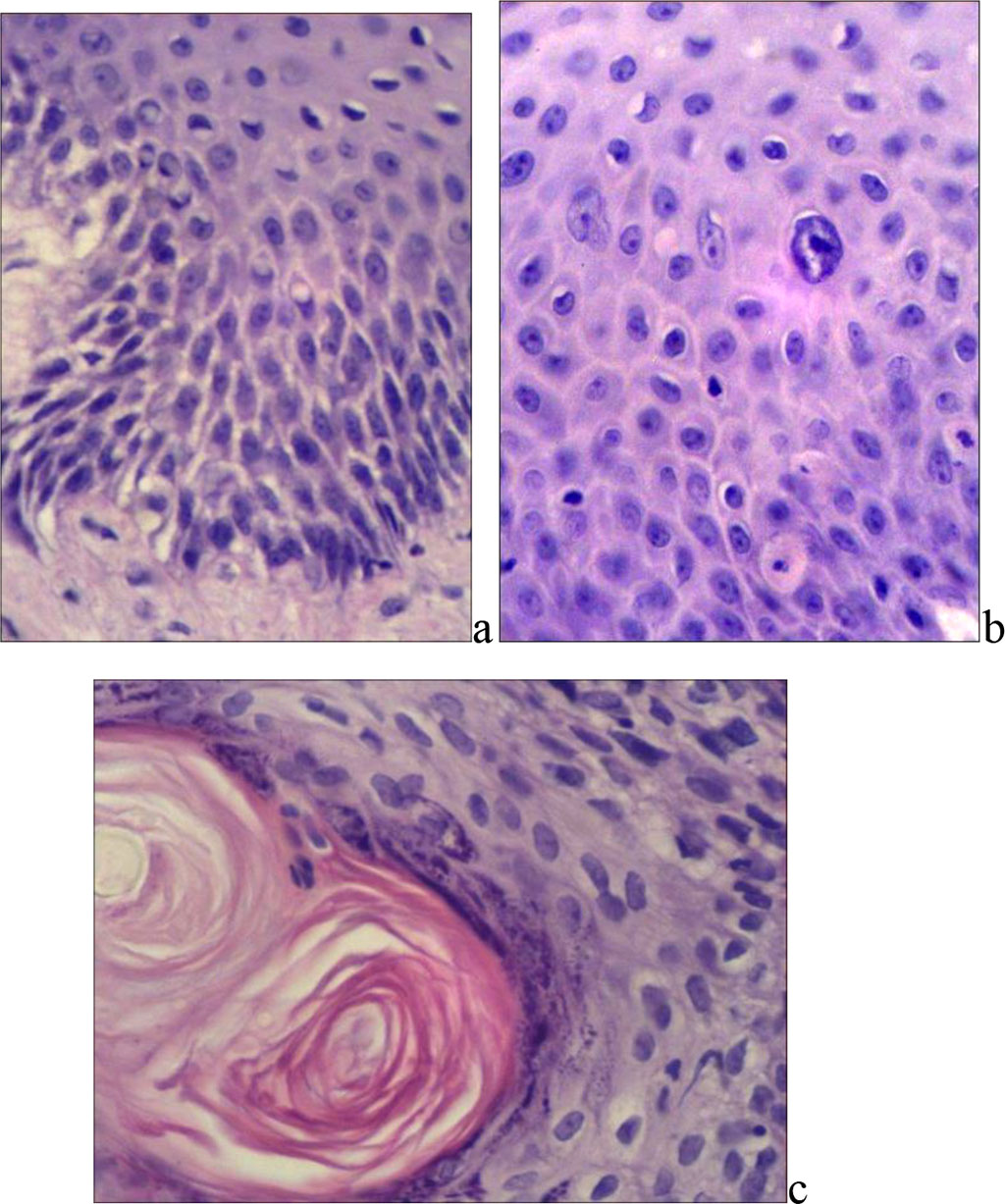
Fig. 1. Differentiated VIN: a) and b) – increased number of prematurely differentiated keratinocytes (staining with hematoxylin and eosin); c) – hyperkeratosis, formation of horny pearls (X400, b) – X600).
In all VIN forms, the subbasal specific volume of microcirculatory bed vessels in VIN averaged 0.1081*0.0094 (Fig. 2).
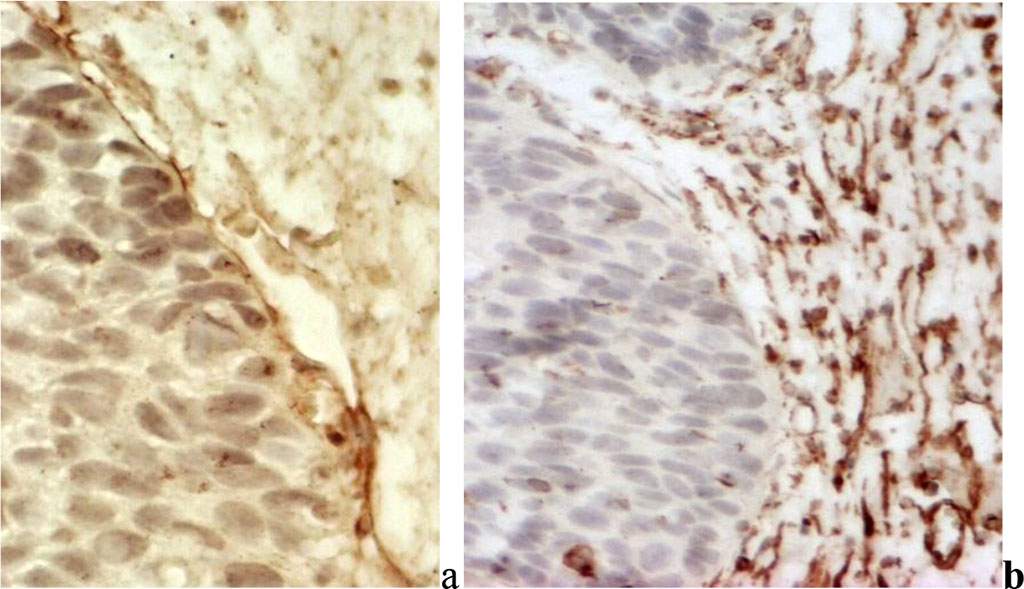
Fig. 2. Differentiated vulvar intraepithelial neoplasia: a) in the basement membrane there is clear intensive expression of type IV collagen; b) a developed subbasal network of microcirculatory vessels is detected;
VIN1 showed basal cell hyperplasia with hyperchromic nuclei containing one to three nucleoli. There were some cells with large round nuclei, hypochromic with finely dispersed chromatin or hyperchromic. The cytoplasm was eosinophilic and optically empty. The cells of the basal layer were perpendicular to the basement membrane.
Moderate cellular polymorphism was observed in the intermediate layer: the cells were round, elongated and irregularly shaped. The nuclei were hyperchromatic or large, light, with delicate chromatin, sometimes optically empty, with one or two nucleoli. There were cells with large hyperchromatic defective nuclei and dense chromatin. Vacuolization of nuclei was observed in single cells. Cells with signs of keratinization were found in the superficial and intermediate sections of the epithelium.
In VIN2, there was disruption of the vertical anisomorphy and stratification of the lower layers of the formation due to basal cell hyperactivity. The cell nuclei were enlarged, often round-oval or elongated. The nuclear-cytoplasmic ratio was shifted towards the nucleus. Most hyperplastic basal and parabasal cells were hyperchromic, but there were single normo- and hypochromic ones.
The cytoplasm of the cells of the lower half of the epithelial layer had a basophilic shade, making it darker than the upper sections. The hyperplastic cells of the lower layers were perpendicular to the basement membrane, the upper layers were horizontal. The number of mitoses was increased, spreading to the cells of the middle third of the epithelial layer. The upper cells retained their normal structure and location.
The lower part of the intermediate layer consisted of hyperplastic basal and parabasal cells, while the upper part consisted of large polygonal cells with round or oval nuclei, finely dispersed chromatin and eosinophilic cytoplasm. In some cases, foci of disordered cell arrangement were observed, forming swirling patterns. The superficial layer sometimes contained flattened cells located parallel to the basement membrane. In nine cases, shallow acanthotic growths were detected. In the capillaries, swelling of the walls and leukocyte stasis were noted, accompanied by inflammatory cells in the vessel walls and connective tissue. Diffuse cellular infiltration of segmented leukocytes was expressed, some of which were PAS-positive, indicating their activity. Neutrophilic infiltration was noted at the border of the epithelial-stromal transition and in the lower layers of the epithelium.
In VIN3, there was disruption of stratification and vertical anisomorphy over a significant part of the epithelium due to disruption of cell maturation and differentiation. Almost the entire epithelial layer was represented by monomorphic, densely located cells of round-oval and elongated shape, similar to basal cells, with the exception of several superficial layers of mature cells with a normal structure. The nuclei of most cells were round or elongated; triangular nuclei were encountered. Most nuclei were hyperchromic with uniform chromatin; normo- and hypochromic nuclei with finely dispersed chromatin were encountered. The nuclear-cytoplasmic ratio was altered in favor of the nucleus.
The cytoplasm of the cells was narrow, almost invisible in places, with a weak basophilic tint. In some vision fields, the cytoplasm was almost absent, forming nuclear territories or naked nuclei. The boundaries of the cells were unclear, cytoplasmic bridges were absent. The number of mitoses was increased. In some cases, morphological manifestations of apoptosis were observed, with individual cells smaller in size than the surrounding ones.
The indicators of proliferative activity of epithelial cells in vulvar intraepithelial neoplasia of varying severity are presented in Table 1.
Table 1. Indicators of proliferative activity of epithelial cells in vulvar intraepithelial neoplasia of varying severity
| Indicator | Severity of vulvar intraepithelial neoplasia | ||
| VIN1 (n=4) | VIN2 (n=4) | VIN3 (n=6) | |
| Mitotic index, ‰ | 3,10±0,68 | 4,63±0,52 | 9,24±0,87 |
| Nuclear-cytoplasmic ratio | 0,33±0,08 | 0,49±0,05 | 0,61±0,09 |
| Average length of intersection of epithelial cell nuclei, µm | 7,21±1,6 | 7,43±1,7 | 8,01±3,6 |
Analysis of the proliferative activity of epithelial cells showed that the average length of intersection of epithelial cell nuclei differed slightly in different VIN types. The nuclear-cytoplasmic ratio differed significantly: 0.33±0.08 in mild VIN and 0.61±0.09 in severe VIN. In VIN1, cells with poor cytoplasm and a ratio of 0.5 or higher (hyperplastic basal cells) occupied no more than a third of the epithelial layer, while the remaining cells had abundant cytoplasm. With increasing severity of VIN, the basal cell activity of the epithelium increased, the percentage of cells with a ratio above 0.5 increased, and in areas of naked nuclei this indicator was almost 1. The mitotic index in VIN3 was 9.24 ± 0.87 ‰, which was 2 times greater than in mild VIN, and almost 3 times greater than in moderate VIN (p < 0.05). Pathological mitoses were observed only in severe VIN. Thus, an increase in the severity of VIN is accompanied by an increase in proliferative activity.
When determining the proliferative potential of epithelial cells, it was found that, with VIN1, positive typing was observed in the cells of the lower third of the epithelial layer, where more than 2/3 of the cells expressed the nuclear proliferation antigen. In one case, the reaction was also detected in individual cells of the intermediate layer. With VIN2, positive typing of the nuclear proliferation antigen was detected in a significant portion of the cells of the basal, parabasal, and intermediate layers. In some areas, cells with a positive reaction to PCNA occupied more than half of the epithelial layer. (Fig. 3).
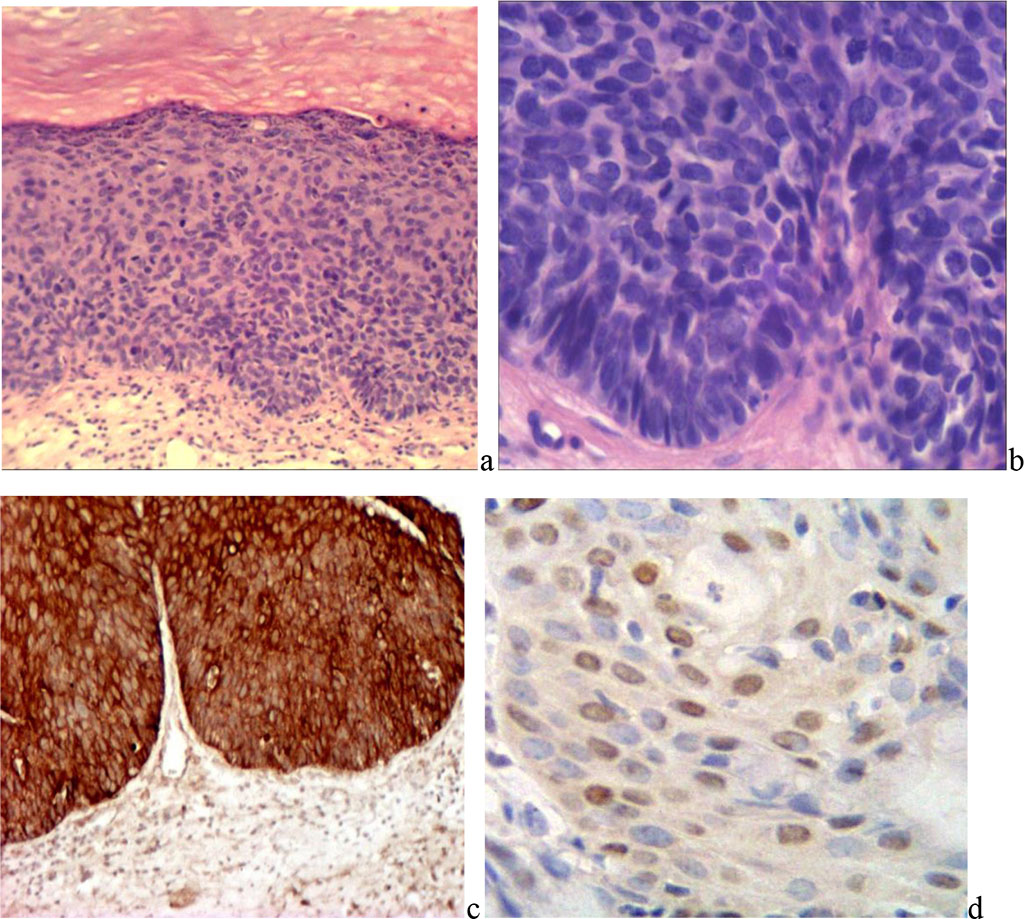
Fig. 3. Vulvar intraepithelial neoplasia: a) mild X200; b) severe X400; c) positive reaction of epithelial cells to pancytokeratin. d) with PCNA MAB X400;
In VIN3, cells of almost all layers of the epithelium expressed nuclear antigen of proliferation. Most cells showed a positive reaction, with the exception of some cells and small groups in the superficial layer. The percentage of cells with a positive reaction to PCNA was on average: VIN1 - 16.0±1.8%, VIN2 - 23.5±2.2%, VIN3 - 31.6±4.1% (p<0.05).
The index of proliferation antigen accumulation in the nuclei of epithelial cells ranged from 15% to 41%, on average for VIN1 – 19.4±1.2%, VIN2 – 28.4±2.3%, VIN3 – 37.5±2.9% (p>0.05).
Immunohistochemical typing of cellular stromal infiltrates revealed a predominance of CD4+ T-lymphocytes, distributed evenly, in places forming small clusters (Fig. 4).
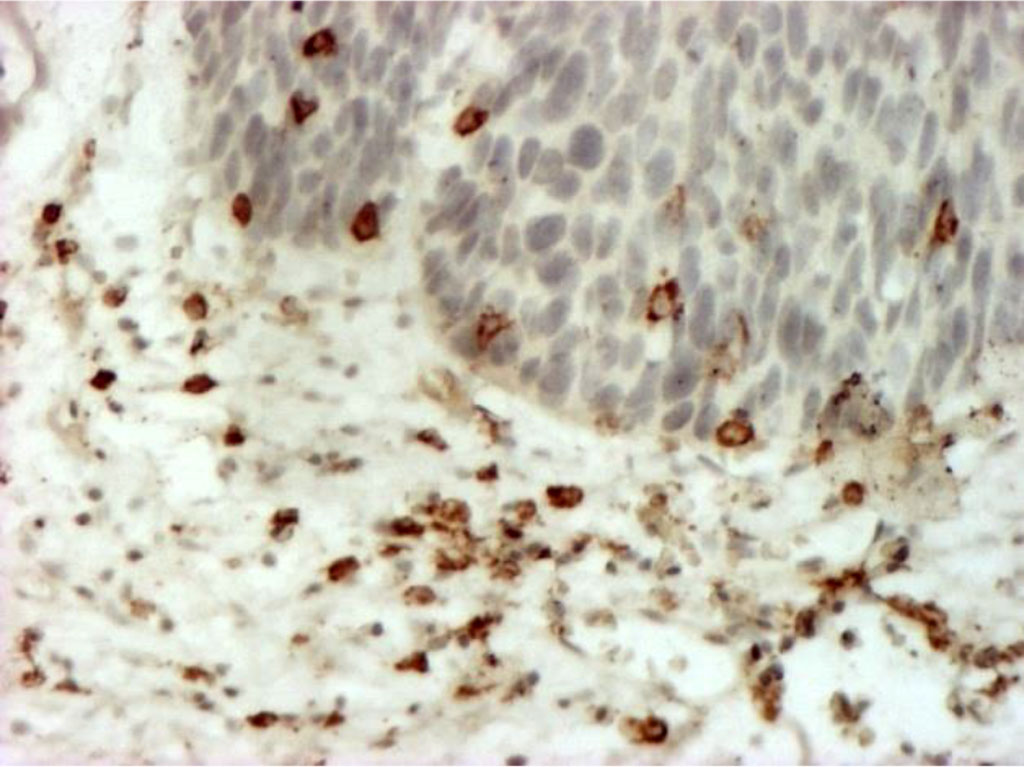
Fig. 4. Stromal lymphocytes (T-helpers) (CD4+), forming small clusters in places. Immunohistochemical staining with MAb to CD4+ X400.
No significant correlation was found between the quantitative and qualitative composition of cellular infiltrates and such factors as the patient's age and the degree of VIN differentiation. For differentiated VIN, the average number of immunocompetent cells per 1 mm² was 13.3±1.6 in the stroma, and 4.7±1.3 within the field of view. Lymphocytes predominated in the infiltrates (87.6±4.9%, p<0.001). Undifferentiated forms of VIN associated with vulvar carcinoma have a low density of immune cellular and inflammatory infiltrates. The quantitative and qualitative composition of cellular infiltrates in VIN1, VIN2 and VIN3 do not have significant intergroup differences (p> 0.05), but can be used to assess the degree of VIN differentiation and differential diagnosis of VIN3 and invasive carcinoma. The results are presented in Table 2.
Table 2. Indicators of quantitative and qualitative composition of cellular infiltrates
| Indicator | Degree of VIN differentiation | ||
| VIN1 (n=4) | VIN2 (n=4) | VIN3 (n=6) | |
| number of cells per 1 mm2 | 14,8±3,5 | 17,1±2,9 | 21,0±5,2 |
| average number of cells in the field of view | 3,1±0,4 | 4,2±0,3 | 6,0±1,1 |
| Cellular composition of the infiltrate, %: | |||
| Lymphocytes | 79,3±4,4 | 85,1±3,6 | 88,9±2,0 |
| Plasmocytes | 8,0±2,3 | 6,3±1,7 | 7,4±0,3 |
| Neutrophils | 6,8±0,4 | 4,3±0,5 | 4,0±0,2 |
| Macrophages | 5,7±0,9 | 5,1±1,1 | 2,2±0,13 |
The 44 cases of primary vulvar carcinomas were distributed according to the degree of cataplasia as follows: a) well-differentiated invasive squamous cell carcinoma (G1) – 12 cases (27.3±7.3%); b) moderately differentiated invasive squamous cell carcinoma (G2) – 15 cases (34.1±7.1%); c) poorly differentiated invasive squamous cell carcinoma (G3) – 17 cases (38.6±7.3%).
Characteristics of well-differentiated (G1) invasive squamous cell carcinoma (SCC) of the vulva: the parenchyma is represented by polymorphic solid complexes of flat polygonal atypical epithelial cells with large hypochromic oval nuclei. Vertical anisomorphy is absent, but small fields of keratohyalin are detected around the cell groups. Keratinization of individual cells with the formation of eosinophilic "cancer pearls" is noted in the complex. The cytoplasm of the cells is light, slightly basophilic, with clear boundaries. Cellular and nuclear polymorphism is weakly expressed. The nuclei are large, oval and round, hypochromic, contain 1-3 hyperchromic nucleoli. The chromatin is finely dispersed and evenly distributed. The nuclear-cytoplasmic ratio is increased. There are few pathological mitoses, but many cells in apoptosis. Epithelial complexes are located among bundles of loose fibrous connective tissue (Table 3).
Table 3. Indicators of the average length of tumor cells nuclear intersection, nuclear-cytoplasmic ratio and proliferative activity of tumor cells in well-differentiated squamous cell carcinoma of the vulva
| Indicator | Value of the indicator (M±m) |
| Average length of intersection of tumor cell nuclei, µm | 5,37±0,29 |
| Nuclear-cytoplasmic ratio (NCR) | 0,61±0,11 |
| Mitotic index, ‰ | 9,48±0,34 |
The basement membrane of atypical epithelial complexes of well-differentiated squamous cell carcinoma of the vulva is unevenly thickened, compacted and represented by eosinophilic, picrinophilic, PAS-positive fibers with double refraction and dichroism in polarized light. There are foci of disorganization of the basement membrane, loosening, decreased metachromasia and a decrease in the content of glycoproteins.
Collagen fibers of the vulvar SCC stroma form dense eosinophilic bundles with uneven accumulation of glycoproteins and non-sulfated glycosaminoglycans, indicating hyalinosis. In some areas, the fibers are loosened, frayed, with altered tinctorial properties, thin, tortuous, with slight basophilia and weak birefringence. In these areas, there is focal proliferation of fibroblasts and small accumulations of macrophages.
Characteristics of moderately differentiated (G2) invasive squamous cell carcinoma (SCC) of the vulva: signs of vertical anisomorphy and keratinization are sharply reduced. The cytoplasm of tumor cells is light, basophilic, PAS-positive, contains glycosaminoglycans and neutral mucopolysaccharides. The nuclei are oval and round, hypochromic, with one to three hyperchromic nucleoli.
Parenchyma cells in moderately differentiated SCC of the vulva vary in size and shape. Polygonal cells with light, weakly eosinophilic cytoplasm and ovoid nuclei with uneven distribution of chromatin predominate at the periphery of tumor complexes. The ratio of nucleus to cytoplasm is approximately equal. Near the basement membrane, large tumor cells have elongated, sharply hyperchromatic nuclei occupying almost the entire volume of the cell.
Moderately differentiated SCC is characterized by inflammatory hyperemia, stromal edema, cellular reaction, hemorrhages and necrosis with perifocal fibrinous and fibrinous-purulent inflammation. There is no stratification of the epithelium. Moderate cataplasia, polymorphism and discomplexation of cells, destruction of the basement membrane are expressed. The ratio of stroma and parenchyma is variable. The stroma consists of thin fibrils of collagen fibers with an uneven content of mucopolysaccharides and glycosaminoglycans, with a low degree of anisotropy and weak birefringence, but clear dichroism.
Characteristics of poorly differentiated (G3) invasive squamous cell carcinoma (SCC) of the vulva: epithelial layers are formed from small atypical cells, signs of vertical anisomorphism and keratinization are absent. An increase in the mitotic index is accompanied by an increase in the number of atypical mitoses. Tumor emboli are detected in ectatic lymphatic and blood vessels, promoting metastasis (Table 4).
Table 4. Morphometric indicators of parenchyma and stroma of vulvar cancer with different degrees of cataplasia
| Morphometric indicators | Invasive squamous cell carcinoma (SCC) of the vulva | ||
| G1 (n=12) | G2 (n=15) | G3 (n=17) | |
| Specific volume of parenchyma | 0,3423±0,0129 | 0,3679±0,0181 | 0,3925±0,0196 |
| Specific volume of stroma | 0,1806±0,0102 | 0,1467±0,0169 | 0,1327±0,0160 |
| Specific volume of vessels | 0,1209± 0.0163 | 0,1143±0,0103 | 0,1316±0,0187 |
| Vascularization coefficient | 0,3532±0,0194 | 0,3107±0,0142 | 0,3352±0,0256 |
| Specific volume of necrotic foci | 0,0342±0,0106 | 0,0582±0,0166 | 0,0813±0,0127 |
| Mitotic index in ‰ | 14,98±0,82 | 17,86±0,90 | 19,73±0,70 |
| Apoptotic index in ‰ | 5,16±0,45 | 4,74±0,62 | 5,08±0,31 |
Comparison of the structure of SCC of the vulva showed that the specific volume of tumor parenchyma significantly increases with an increase in the degree of differentiation: 0.0196 (G3) (p±0.0181 (G2) and 0.3925±0.0129 (G1), 0.3679± 0.3423<0.0160) (p±0.0169; in G3 – 0.13270.0102; in G2 – 0.1467±0.005). The specific volume of stroma varies widely both within different tumors and within a single neoplasm (in G1 – 0.1806<0.05).0.0127 (p±0.0166; in G3 – 0.0813±0.0106; in G2 – 0.0582±0.0123.
The high specific volume of the vascular bed in squamous cell carcinoma of the vulva determines high values of the vascularization coefficient. However, a high specific volume of necrotic foci is also revealed in the tumor tissue: in G1 - 0,0342±0,0106. This is explained by dystrophic changes in the walls of the microcirculatory bed, such as mucoid swelling and fibrinoid changes, confirmed by a positive PAS reaction and pronounced metachromasia when stained with toluidine blue at pH 5.3. A high frequency of mixed and agglutinative thrombi is observed in the vessels of the microcirculatory bed near the necrotic foci.
Qualitative and quantitative characteristics of cellular infiltrates in the stroma of well-, moderately and poorly differentiated invasive squamous cell vulvar carcinoma de novo are presented in Table 5.
Table 5. Qualitative and quantitative characteristics of cellular infiltrates in the stroma of well-, moderately and poorly differentiated invasive squamous cell carcinoma of the vulva de novo
| Indicator | Degree of tumor differentiation | ||
| G1 (n=12) | G2 (n=15) | G3 (n=17) | |
| number of cells per 1 mm2 | 34,8±4,5 | 47,1±7,4 | 53,6±8,1 |
| average number of cells in the field of view | 6,1±0,7 | 8,2±0,6 | 9,0±1,2 |
| Cellular composition of the infiltrate, %: | |||
| Lymphocytes | 89,4±3,5 | 91,0±4,5 | 93,1±1,1 |
| Plasmocytes | 3,1±0,1 | 1,7±0,3 | 1,1±0,5 |
| Neutrophils | 3,6±0,4 | 3,8±0,2 | 4,0±0,29 |
| Macrophages | 4,8±0.2 | 4,9±0,8 | 2,2±0,13 |
Qualitative and quantitative characteristics of cellular infiltrates in the stroma of well- (G1), moderately (G2) and poorly (G3) differentiated invasive squamous cell carcinoma of the vulva show a low density of immunocompetent cells, which indicates a weak immune response to tumor progression. Lymphocytes predominate in stromal infiltrates: 89.4%±3.5% (G1), 91.0%±4.5% (G2), 93.1%±1.1% (G3). Single plasma cells and a small number of neutrophilic leukocytes are also found: from 3.6%±0.4% (G1) to 4.0%±0.29% (G3) (p>0.05). To summarize, the percentage of cells with a positive reaction to PCNA varied: VIN1 – 16.0±1.8%, VIN2 – 23.5±2.2%, VIN3 – 31.6±4.1% (p<0.05). The index of accumulation of proliferation antigen in the epithelial cells nuclei: VIN1 – 19.4±1.2%, VIN2 – 28.4±2.3%, VIN3 – 37.5±2.9% (p>0.05). The average length of intersection of tumor cell nuclei is 5.37±0.29 μm. The nuclear-cytoplasmic ratio is 0.61±0.11, indicating developed cytoplasm. The mitotic activity of cells is on average 9.48 ‰±0.34 ‰.
Comparative morphological assessment of the structure of vulvar SCC shows high values of the specific volume of tumor parenchyma: G1 – 0.3423±0.0129, G2 – 0.3679±0.0181, G3 – 0.3925±0.0196 (p<0.05). The specific volume of necrotic foci is also high: G1 – 0.0342±0.0106, G2 – 0.0582±0.0166, G3 – 0.0813±0.0127 (p<0.05). Cellular infiltrates of the stroma of invasive squamous cell carcinoma are dominated by lymphocytes: G1 – 89.4%±3.5%, G2 – 91.0%±4.5%, G3 – 93.1%±1.1%, with a small number of plasma cells and neutrophilic leukocytes: G1 – 3.6%±0.4%, G3 – 4.0%±0.29% (p>0.05).
Primary invasive squamous cell carcinoma of the vulva (invasive SCC) is characterized by pronounced signs of mitotic activity, multiple pathological mitotic figures and delay in metaphase. The mitotic index is: G1 – 14.98±0.82‰, G2 – 17.86±0.90‰, G3 – 19.73±0.70‰ (p<0.05). In VIN3, this indicator is 9.24±0.87‰ and significantly differs from G1 (p<0.05). This difference is important for differential diagnosis between VIN3 and invasive SCC (G1), as well as for determining the extent of surgical intervention.
These data confirm that the degree of differentiation and proliferative activity play a key role in the prognosis and treatment of vulvar cancer. For a more accurate diagnosis and effective treatment, it is necessary to take into account these morphological and molecular features.