- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2023. 13; 5: e1. DOI 10.35630/2023/13/5.503
The aim of this study was to experimentally validate the possibility and advisability of the treatment of polypropylene mesh implants with xenogenic cerebrospinal fluid in order to reduce a local acute inflammatory response. An experimental evaluation of the effectiveness of the proprietary method of polypropylene mesh implant processing during their implantation in the tissues of the anterior abdominal wall (Patent of Ukraine No. 146637) is presented. It has been proven that the use of the proposed new technology for the treatment of polypropylene mesh implants with a biocompatible component is a simple, safe and effective method that optimizes the reparative processes in the peri-implant zone without affecting the mechanical properties of mesh implants. All of the treatment effects were found to increase the biocompatibility and improve the survival rate of mesh implants. The thickness of the inflammatory ridge around the elements of mesh implants significantly decreased on average by 35.3% when xenogenic cerebrospinal fluid was used.
Keywords: polypropylene, anterior abdominal wall, mesh implants, xenogenic cerebrospinal fluid
Today, more than 300 different types of plastic materials for hernioplasty are produced in the world, and their number increases annually due to attempts to create an “ideal” mesh implant (MI) [1, 2]. The implantation of MI into soft tissues causes the development of an inflammatory reaction in the recipient's body similar to a typical foreign body reaction [3, 4]. The aim of the proposed process is to insulate the foreign body from the recipient tissue by forming an artificial environment around the implant [5, 6].
The use of some MI surface coating agents is able to mask the foreign surface by creating a hydrophilic space, which improves the interaction between MI and the host tissues, increases the functionality and survival of MI [7]. At present, various natural and synthetic materials have been proposed for this purpose. Materials of natural origin include embryonic fibroblasts, mesenchymal stem cells, cell-free xenograft, and deproteinized dialysate from calf blood [8, 9, 10, 11, 12, 13]. The possibility of using xenopericardium [14], amniotic membrane [15], xenoperitoneal extracellular matrix [16], bacterial nanocellulose [17], and autoplasma [18] has been widely studied. Thus, the search for new ways to improve the biocompatibility of MI remains an urgent problem of modern herniology, indicating the relevance of this topic and the need for further research in this area [12, 19]. One possible agent for coating the surface of MI could be xenogeneic cerebrospinal fluid (CSF). A unique feature of CSF is the absence of interspecies immunological incompatibility, which allows for the use of the available bovine CSF to manufacture xenogenic biological products [20].
The aim of the study: to experimentally validate the possibility and the scale of the xenogenic CSF application in the treatment of polypropylene (PP) MI to reduce the manifestations of the local inflammatory reaction.
The study was carried out on 24 white nonlinear male rats weighing 200–250g. The animals were kept in the vivarium of the Department of Human Anatomy of the Medical Academy named after V.I. SI Georgievsky in compliance with the rules and international recommendations of the European Convention for the Protection of Animals (1997). The experiment was carried out in compliance with the norms of humane treatment, in accordance with the current legislation on working with laboratory animals, and was approved by the local commission on bioethics.
All animals, under ether anesthesia, were implanted with PP-MI in the anterior abdominal wall (AAW) tissue. PP mesh “Alfa Vita 90” (Ukraine) in strips up to 1cm² was used as MI. The material was fixed in the AAW tissues with a nylon ligature from four sides. Then the wound was sutured and the integrity of the AAW was restored. The animals were divided into two groups. The main group (MG, n=12) consisted of animals that were implanted with PP-MI after treatment with xenogenic CSF [21]. The latter was prepared traditionally. The exposure time was 10 minutes. The control group (CG, n=12) consisted of animals that were implanted with MI without pretreatment.
The animals were withdrawn from the experiment on Days 7, 14, 21, and 28 after implantation. After fixation in 10% neutral formaline, histological sections were prepared from the preparations according to the standard technique. Sections were stained with H&E, followed by histological and histomorphometric examination.
The results were evaluated using the Master of Morphology and SPSS computer programs.
According to the data of the study, by Day 7 of the experiment animals of the CG showed a pronounced penetration of leukocytes along the circumference of the PP particles of MI. At the same time, the ontogenesis of granulation tissue along the peripheral areas was observed, accompanied by sporadic lymphocytes and histiocytes (Figure 1).
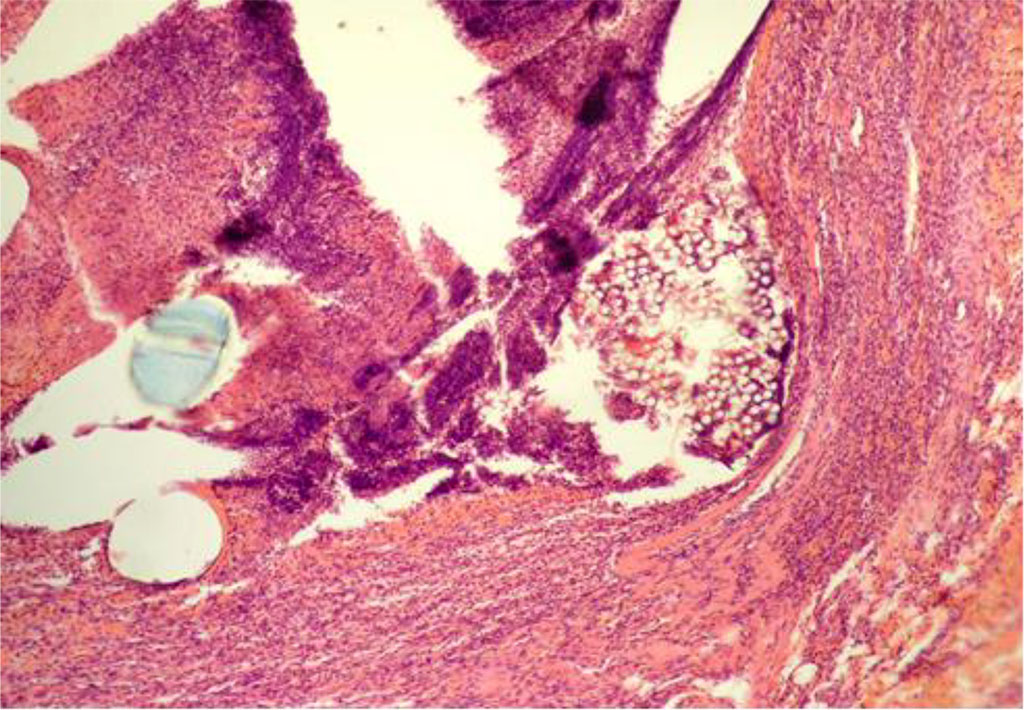
Fig. 1. AAW of the CG animal by Day 7 of the experiment. Staining with H&E. ×10
When measuring the thickness of inflammatory changes (IC) in the circumference of the PP-MI fragments, this indicator was 41.607±0.279 μm (Table 1).
Table 1. The thickness of inflammatory changes (IC) in the circumference of the PP-MI fragments (μm)
| Day of the experiment | CG | MG | P-value |
| Day 7 | 41,607±0,279 | 25,233±0,129 | 0.000 |
| Day 14 | 46,724±0,310 | 21,863±0,128 | 0.000 |
| Day 21 | 40,012±0,283 | 21,593±0,107 | 0.000 |
| Day 28 | 31,068±0,270 | 20,104±0,196 | 0.000 |
In the main group, the nonspecific transformations were observed by Day 7 of the experiment, characterized by restrained manifestations of plethora and edema. A limited number of macrophages and solid fibroblasts were visualized adjacent to the PP-MI fragments. In addition, macrophages were characterized by the presence of enlarged, excellently stained nuclei. Further, a moderate number of both leukocytes and lymphocytes was determined. By this time, the elements of the new loose connective tissue and capillaries of the granulation tissue were already observed (Figure 2).
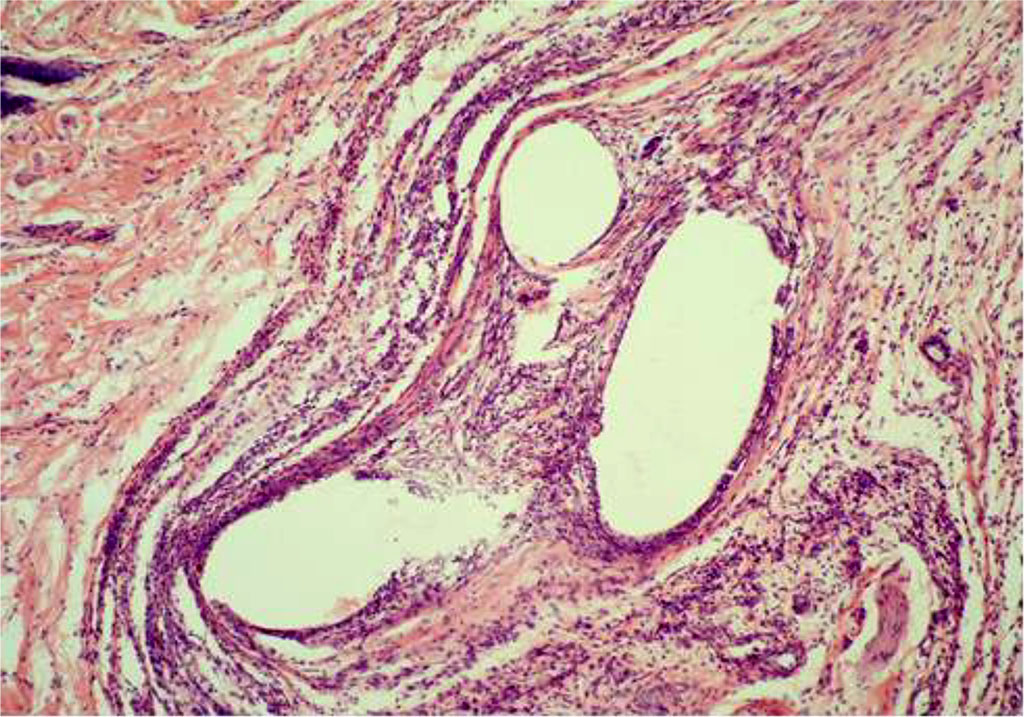
Fig. 2. AAW of the MG animal by Day 7 of the experiment. Staining with H&E. ×10
An average value of 25.233±0.129 μm of inflammatory changes in the circumference of PP-MI fragments was established, which was significantly less than in the control group animals.
By Day 14 of the experiment, the animals of the CG were diagnosed with a decrease in the population of leukocytes and an increase in the number of histiocytes and lymphocytes. At the same time, sporadic siderophages were visualized, as well as giant cells of phagocytosis (Figure 3). The thickness of IC in the circumference of the PP-MI fragments was 46.724±0.310 μm.
By Day 14 of the experiment, there was an absolute disappearance of the phenomena of plethora and edema in the main group.

Fig. 3. AAW of the CG animal by Day 14 of the experiment. Staining with H&E. ×10
The dimensions of the inflammation zone significantly decreased over time when compared with the same presentation in the previous observation periods. There was a decrease in the number of segmented leukocytes. On the other hand, the number of lymphocytes did not change. The number of macrophages also decreased to some extent, while their average size increased. An increased number of connective tissue fibers and fibroblasts was visualized adjacent to the PP-MI, while the giant cells of phagocytosis were not identified (Figure 4). During this observation period, an average value of the extent of the inflammatory changes in the circumference of PP-MI fragments measured at 21.863±0.128 μm.
By Day 21, in the CG, the inflammatory manifestations were more pronounced than in the MG. Giant cells of phagocytosis were detected near the PP-MI particles (Figure 5). The thickness of IC in the circumference of the PP-MI fragments was 40.012±0.283 μm.
By Day 21, a decrease in the number of lymphocytes and macrophages was noted in the main group. The segmented leukocytes were not found in the structure of the inflammatory infiltrate. An extremely limited giant cell response was observed around the PP-MI. There was an increased amount of new connective tissue, starting to have the characteristic appearance of clutches that are woven into the structures of PP-MI, while the thickness of the perifocal inflammatory belt continued to decrease (Figure 6). An average thickness of inflammatory changes in the circumference of PP-MI fragments was 21.593±0.107 μm during this period of the experiment.
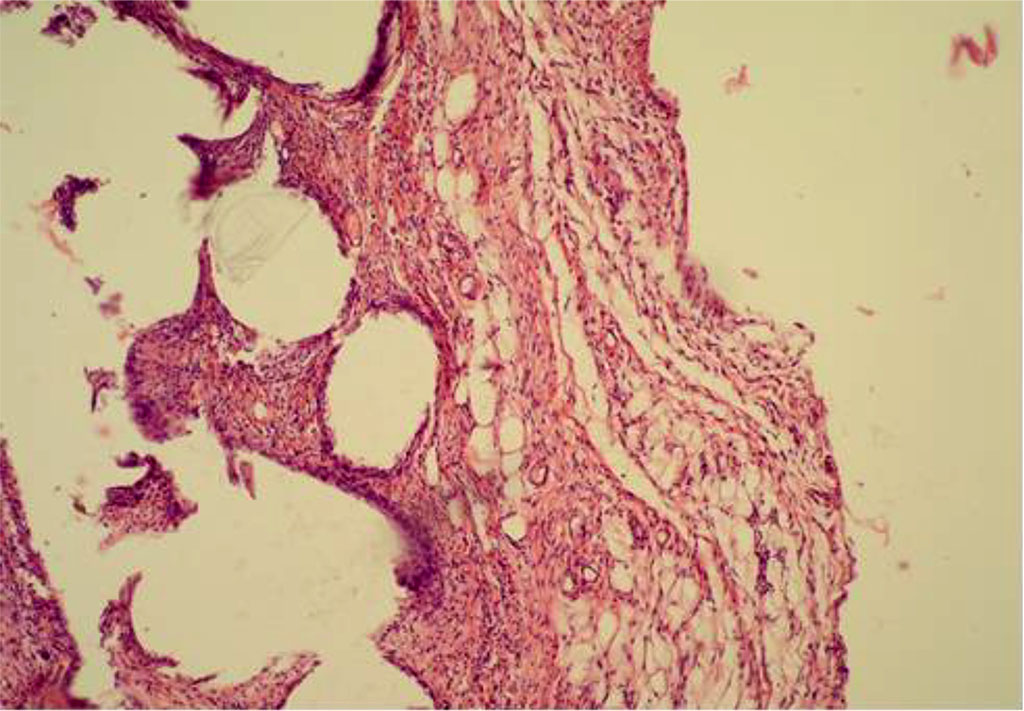
Fig. 4. AAW of the MG animal by Day 14 of the experiment. Staining with H&E. ×10
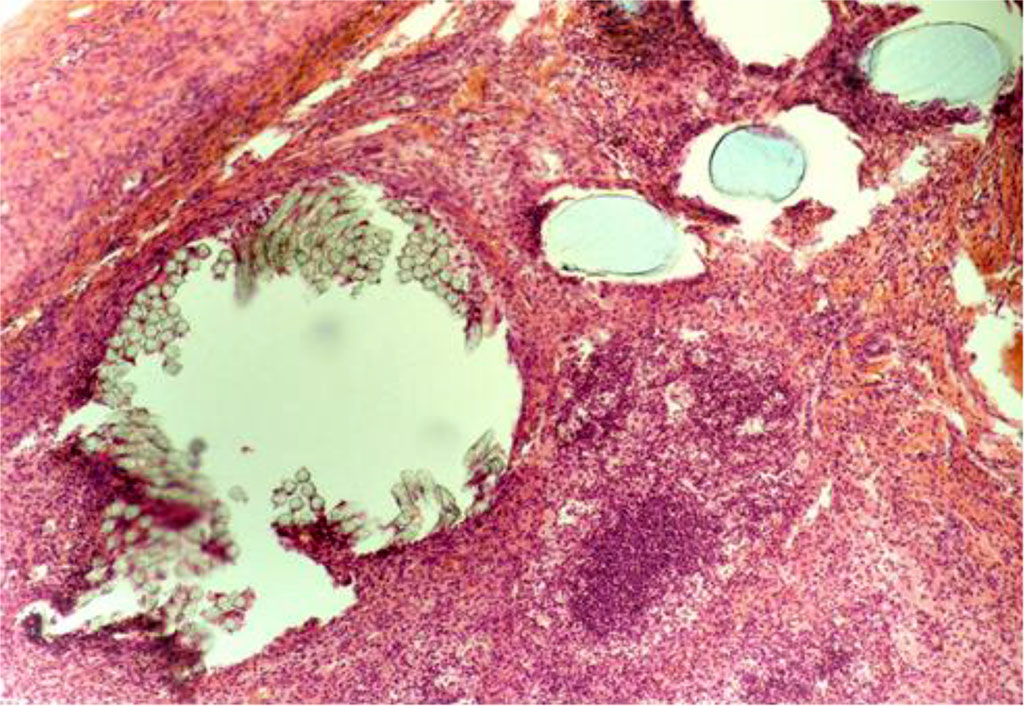
Fig. 5. The fragment of AAW of the CG animal with elements of PP-MI. Day 21 of the experiment. Staining with H&E. ×10

Fig. 6. The fragment of AAW of the MG animal with elements of PP-MI. Day 21 of the experiment. Staining with H&E. ×10
By Day 28, in the CG, intense inflammation continued to be recorded near the PP-MI filaments. Both neutrophils and lymphocytes with giant cells of phagocytosis, as well as macrophages, were visualized, as well as a significant number of siderophages (Figure 7). The thickness of IC in the circumference of the PP-MI fragments was 31.068±0.270 μm.
By Day 28, the thickness of the inflammatory changes in the circumference of the PP-MI fragments in the main group was 20,104±0,196 μm. By this time, there was a decrease in the number of lymphocytes as well as macrophages. Sporadic giant cells are observed, which were identified in some areas of micropreparations (Figure 8).
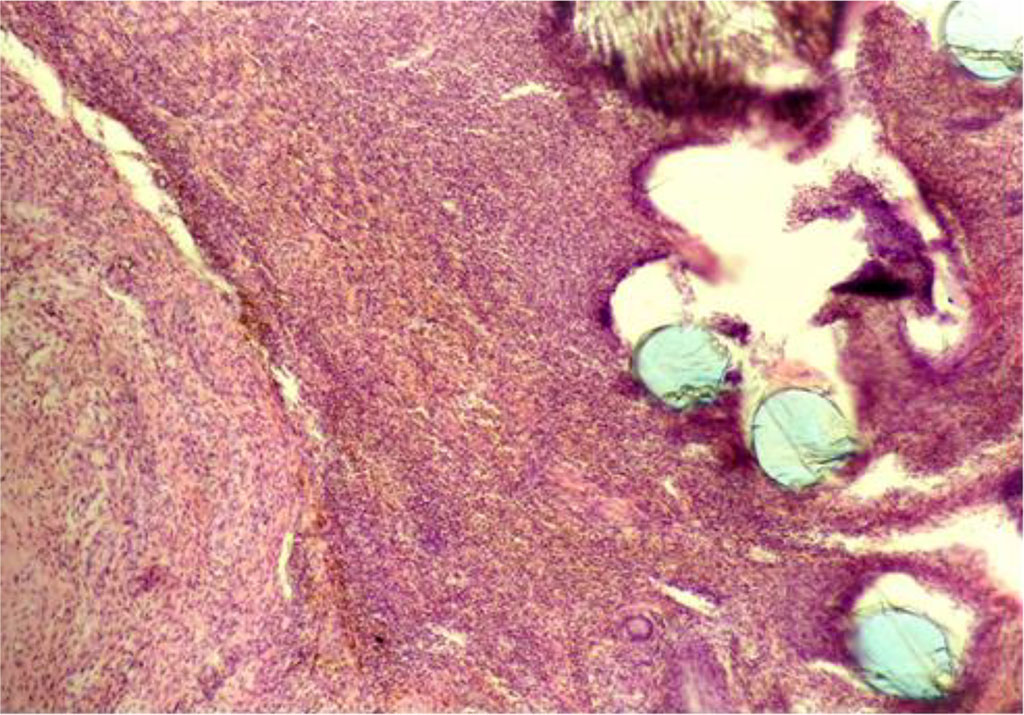
Fig. 7. The fragment of AAW of the CG animal with elements of PP-MI. Day 28 of the experiment. Staining with H&E. ×10
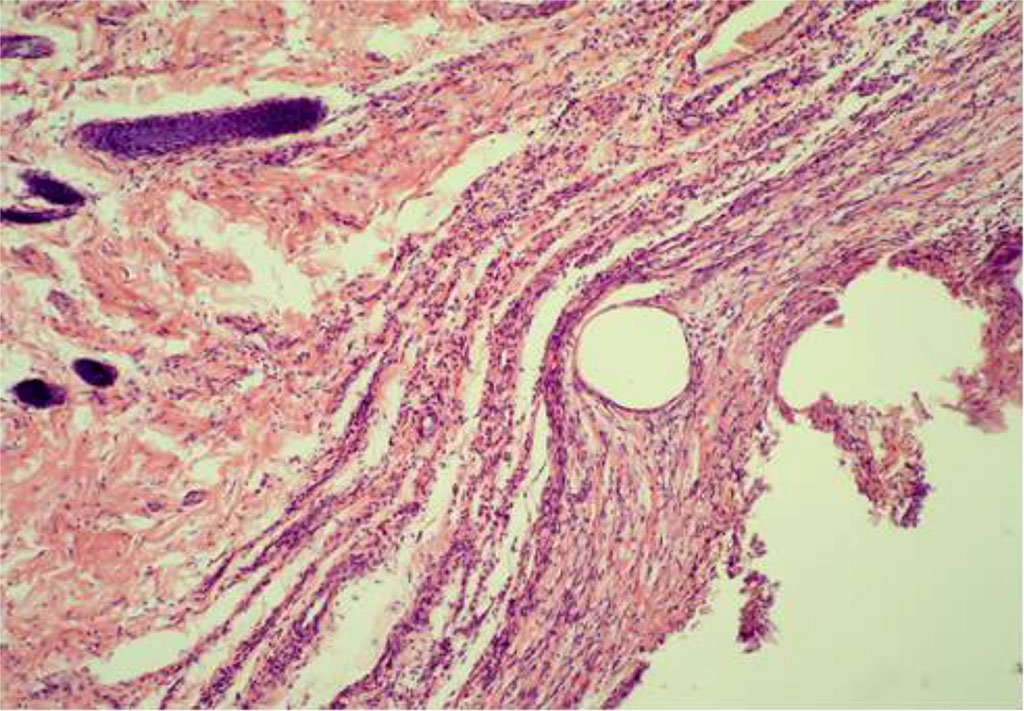
Fig. 8. The fragment of AAW of the MG animal with elements of PP-MI. Day 28 of the experiment. Staining with H&E. ×10
The presented results of histological and morphometric studies confirm that PP-MI implantation causes a local aseptic inflammatory response. However, its intensity differed significantly in different experimental groups of animals during all periods of the research experiment. The greatest local aseptic inflammatory response to PP-MI implantation was observed in the control group, and this was true for all periods of observation. The inflammatory reaction of a much lesser extent was observed in animals of the main group.
It is known that the MI surface is the first to come into contact with the body, and it causes a cascade of reactions of the cellular to humoral immunity. Due to the electronic heterogeneity of the surface layer on the MI, there are centers that can be recognized by the immune system of the body and lead to adhesion of its corresponding elements, which, in turn, mobilizes its protective properties in the form of local aseptic inflammation [22]. The proposed anti-inflammatory agent acts by creating a thin monolayer of CSF that is adsorbed on the surface of MI, thus covering the possible sites of binding of the elements of the immune system. Thus, the technique of treating PP-MI with an anti-inflammatory agent was experimentally validated, and the bovine CSF was proposed for this purpose. The findings of the performed microscopic studies showed the effectiveness of the proposed technology for reducing the local inflammatory response during PP-MI implantation in the AAW tissues. The presented proprietary technique of treating PP-MI with an anti-inflammatory agent can be used in herniological practice since it is easily reproducible and its implementation does not require significant investments. Nevertheless, the legal aspects of the proposed technique shown in this experiment remain a cardinal barrier for its subsequent application in the clinical setting, namely, obtaining permits (licenses) for their testing in clinical trials.
In this research experiment, the treatment of PP-MIs with the xenogenic CSF created favorable conditions for their full integration into the musculoaponeurotic layer of AAW tissues. When applying the xenogenic CSF, the thickness of inflammatory changes in the circumference of MI fragments decreased on average by 35.3%, which was statistically significant (p<0.05).