- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.8
The aim of this study was to determine the role of surgical stress on the formation of p53-positive and dark neurons (DN) in the hippocampus, and to examine the parallelism of their formation in the pyramidal layer of the hippocampus.
Simulated septoplasty was performed on 20 Wistar rats. The hippocampus and dentate gyrus (DG) were examined, in which the number of DN and p53-positive neurons was determined at 2, 4 and 6 days after surgery.
Compared with the control group (n = 5), the number of DN and p53-positive neurons increased in experimental animals at all periods. A direct relationship was obtained between the increase in the number of DN and p53-positive neurons in the hippocampal formation.
Septoplasty simulation in rats results in the pathogenetic cascades onset, which, in its turn, changes the morpho-functional properties of neurons of the pyramidal layer of the hippocampus and contributes to their neuroplasticity. Activation of NMDS receptors of neurons during stress, apparently, initiates two ways of neuron life – the beginning of p53 protein expression and the DN formation. Both ways can finally lead to apoptosis. The formation of dark neurons and the expression of the p53 protein in them are most likely to be interconnected and can probably provide neuroprotective mechanisms.
Keywords: septoplasty, stress, p53, dark neurons, hippocampus.
Simulated septoplasty in rats leads to the development of a powerful stress response. Few experimental data are available to determine the consequences of nasal surgery [1].
Various stressors lead to changes in the functional state of neurons with the development of subsequent morpho-physiological abnormalities [2]. The hippocampus receives special attention in stress because it is very sensitive to various damaging factors [3, 4]. In neuronal damage, as in damage to other cells, p53 protein is an activator of transcription of a specific set of target genes, a cell cycle inhibitory regulatory factor and an effector of cellular responses to damage, which include cell cycle arrest and apoptosis [5]. However, p53 has also been shown to be neuroprotective. For example, this has been shown in an in vivo model of tautopathy [6]. P53 controls the transcription of a group of genes involved in the synaptic function of neurons. The transcriptional control of p53 of these synaptic genes is conserved in mouse neurons and the human brain [6]. Morphologically altered neurons after exposure to stressors can have basophilia in their staining [3, 7]. Such neurons are usually referred to as dark neurons. They have specific morphological features: shrunken cytoplasm, karyopiknosis, corkscrew axon [3, 7]. Apoptosis is believed to occur in these neurons [8]. However, it is not excluded that dark neurons are capable of restoring their morpho-functional state under certain conditions [7].
However, no studies evaluating the parallelism of p53 protein expression in hippocampal neurons and the appearance of dark neurons have been performed in a septoplasty simulation in rats.
The aim of this study was to determine the role of surgical stress on the formation of p53-positive and dark neurons in the hippocampus, and to examine the parallelism of their formation in the pyramidal layer of the hippocampus.
In the study, 20 sexually mature male Wistar rats weighing 250±20 g were randomly assigned into experimental (n=15) and control (n=5) groups. The rats were kept under controlled temperature (23±2.5°C), 12-hour illumination and free access to water and food. Anesthesia was administered with zoletil 100 solution (15 mg/kg) 10 min before surgery to 15 rats, which constituted the experimental group.
The animals were housed in a specially equipped room, access to which was limited. Intact animals were housed in cages for individual housing. In the experimental male rats were used no earlier than 2 weeks later – the period of adaptation to new conditions of detention. The rats received a standard diet once a day, with free access to water. All animals during the experiments were under the same conditions. The maintenance of rats, modeling of surgical trauma – septoplasty, as well as the withdrawal of animals from experiments were carried out in accordance with the ethical standards set up in the Geneva Convention "International Guiding principles for Biomedical Research Involving Animals" (Geneva, 1990).
Septoplasty was simulated in 20 animals of the experimental group using the standard method by zigzag scarification of the nasal septum mucosa with a sharp probe in caudo-cranial direction (Fig. 1a)[9].
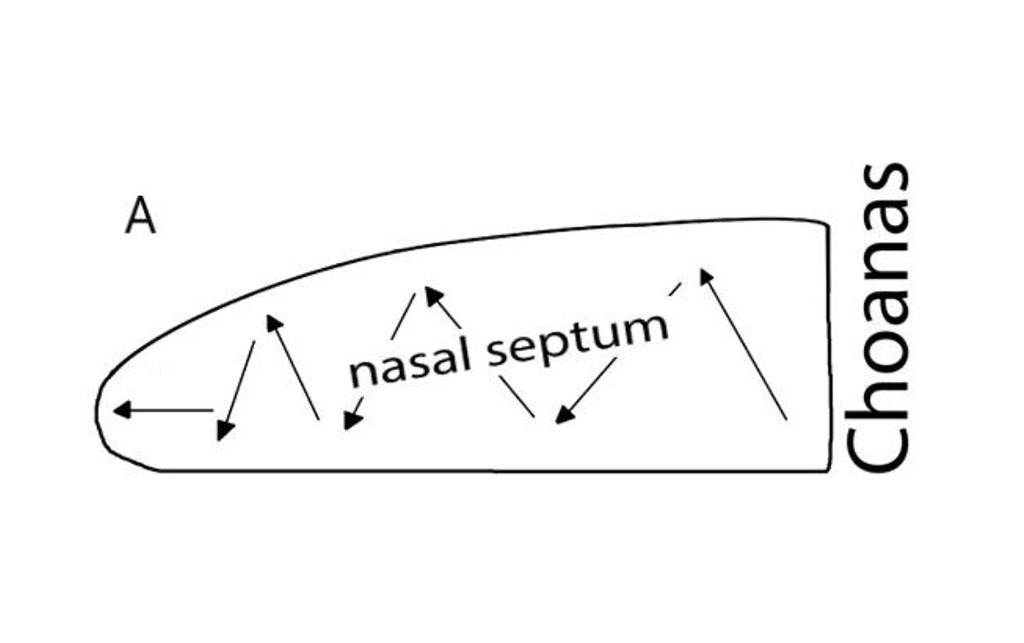
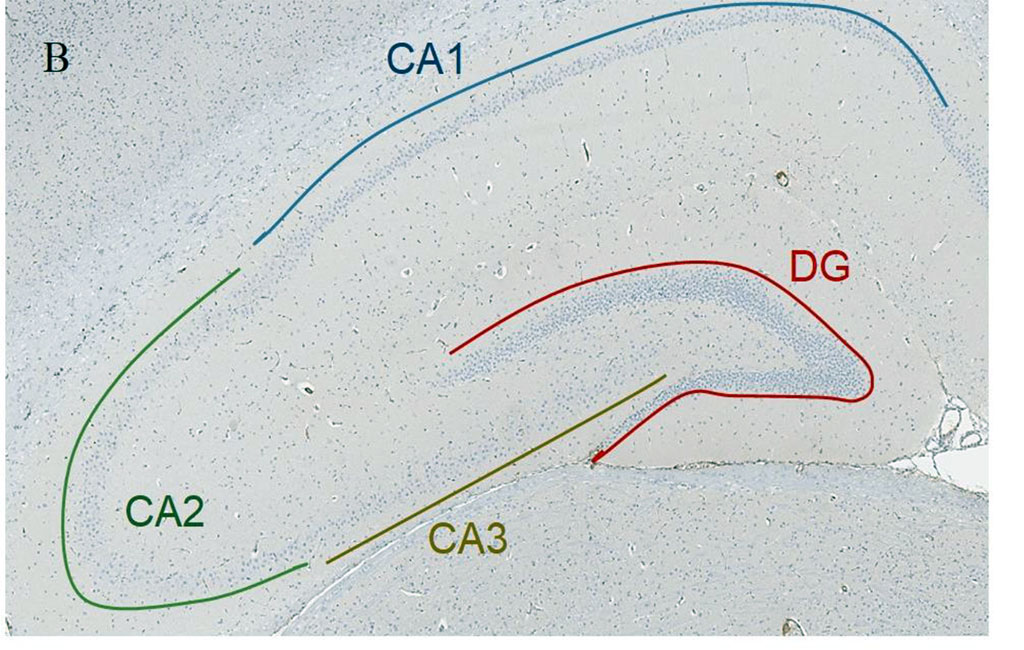
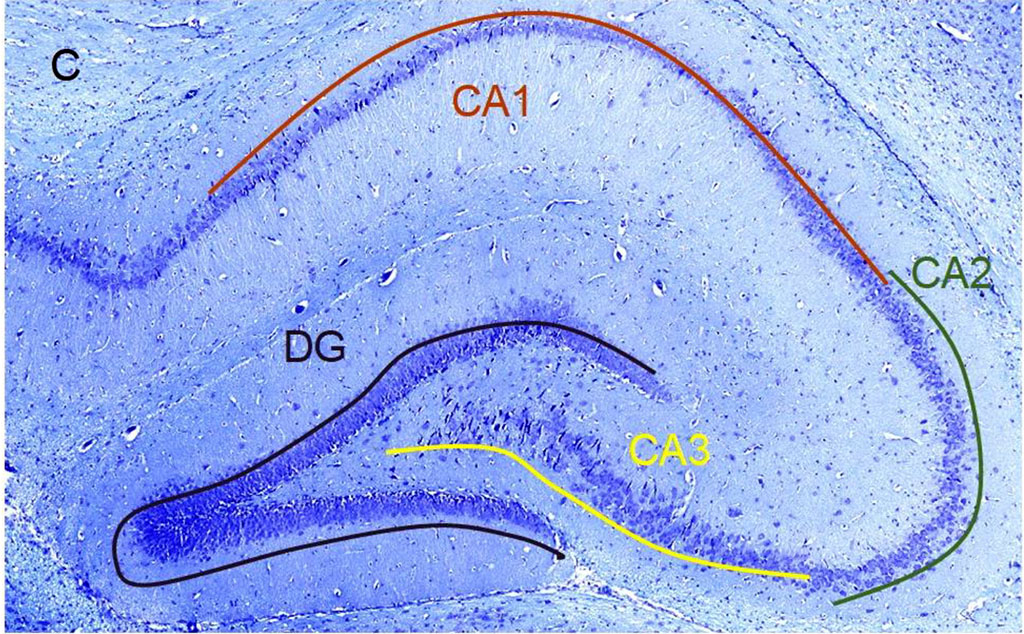
Figure 1. А. Scheme of the septoplasty simulation. Arrows indicate the direction of the nasal septum scarification. B. Location of the rat hippocampus subfields. Immunohistochemical reaction anti-p53. Staining with Mayer's hematoxylin. Magnification, х10. С. Location of the rat hippocampus subfields. Nissl staining. Magnification, х10
In the experimental and control groups, euthanasia was carried out by administering toxic doses of zoletil 100 solution. In the experimental group the animals were slaughtered on the 2nd, 6th and 14th days postoperatively, 5 animals for each period. The brain was first perfused through the aorta with 0.9% sodium chloride solution, then with 10% formalin buffered solution, after which the brains were encased in paraffin blocks. Serial slices of the brain at the level of the bregma were taken with a microtome blade and 8 slices in the frontal plane, 4 µm thick, were obtained from each animal.
In each rat, 10 brain slices were stained with antibodies to p53 protein with Meyer's haematoxylin and 10 slices were stained with Nissl toluidine blue. Hippocampal subfields CA1, CA2, CA3 and dentate gyrus (DG) were studied (Fig. 1b, 1с).
In the pyramidal subfield layer, the absolute number of neurons that were nuclear antibody-positive to p53 protein was counted, as well as the number of dark neurons (Fig. 2). The counting area in each subfield was 20934±1260 µm2. Neurons are counted using the Aperio ImageScope program. For the histological specimen analysis, the ImageJ software was used.
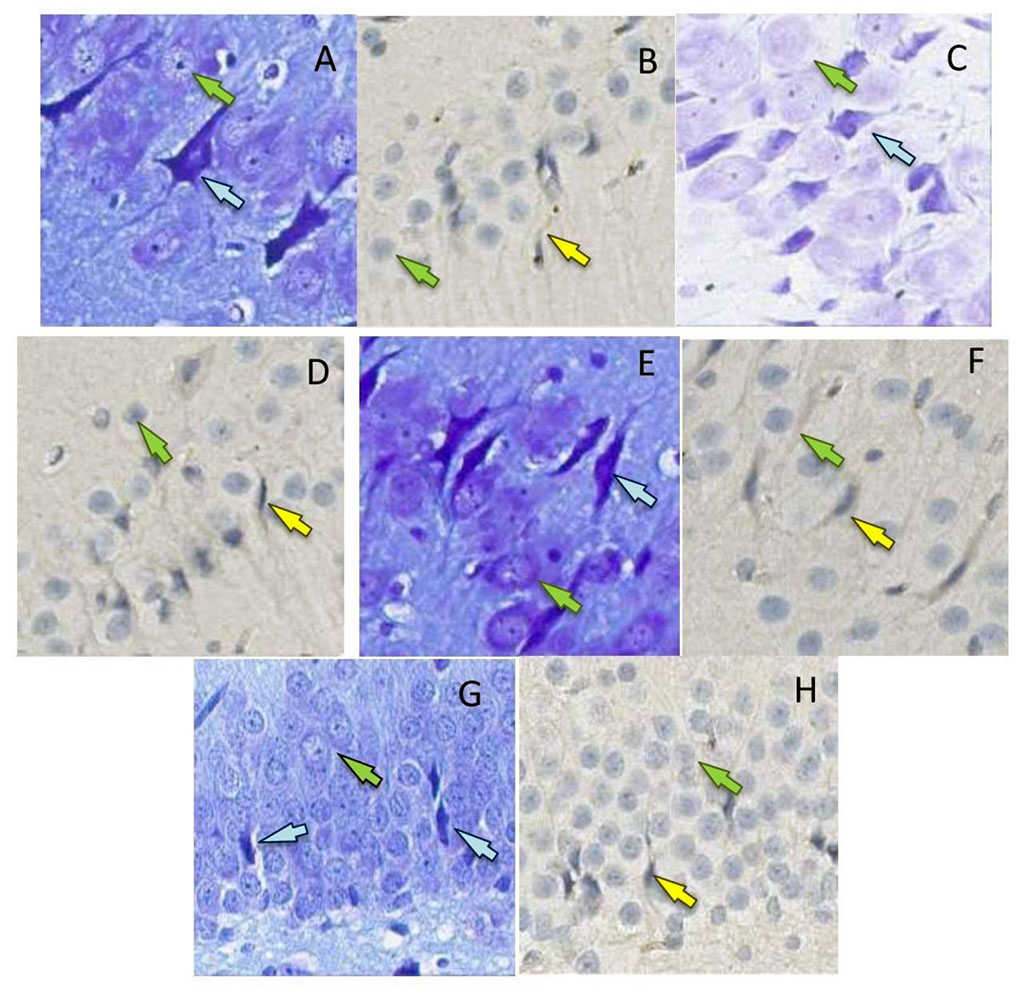
Figure 2. P53-positive neurons (b, d, f, h) (yellow arrows, surrounded by mouse monoclonal antibodies to p53 protein, x400) and dark neurons (a, c, d, g) (blue arrows, Nissl toluidine blue, x400) in the hippocampal formation in rats on the 2nd (a, b, g), 4th (c, d, h) and 6th days (e, f) after the septoplasty simulation. Green arrows indicate intact neurons. Subfield CA1 – a, d; subfield CA2 – b, c; subfield CA3 – e, f; DG – g, h.
The data obtained using cell counting methods were presented as mean ± SE. Then, they were compared between both groups using a t-test SPSS 21software.
There was a non-Gaussian distribution in the number of THs in the pyramidal layer of the hippocampus both in the experimental and control groups. In CA1, the number of TNs on days 2 and 4 did not differ significantly from the control, but on day 6 postoperatively there was a decrease (p<0.001) (Fig.3b). There was no significant difference in CA2 between the experimental group and the control group. On day 2 postoperatively, there was a minimum of THs compared to day 4 (p<0.001). On day 4 postoperatively, there was the peak in number of THs in CA3 compared to the rest of the day (p<0.001). In the control group, the number of THs was not different from day 2, but it was significantly lower compared to day 4 (p<0.001) and day 6 (p<0.05) after surgery (Fig. 3b). In DG, similar results to CA3 were observed (Fig.3b).
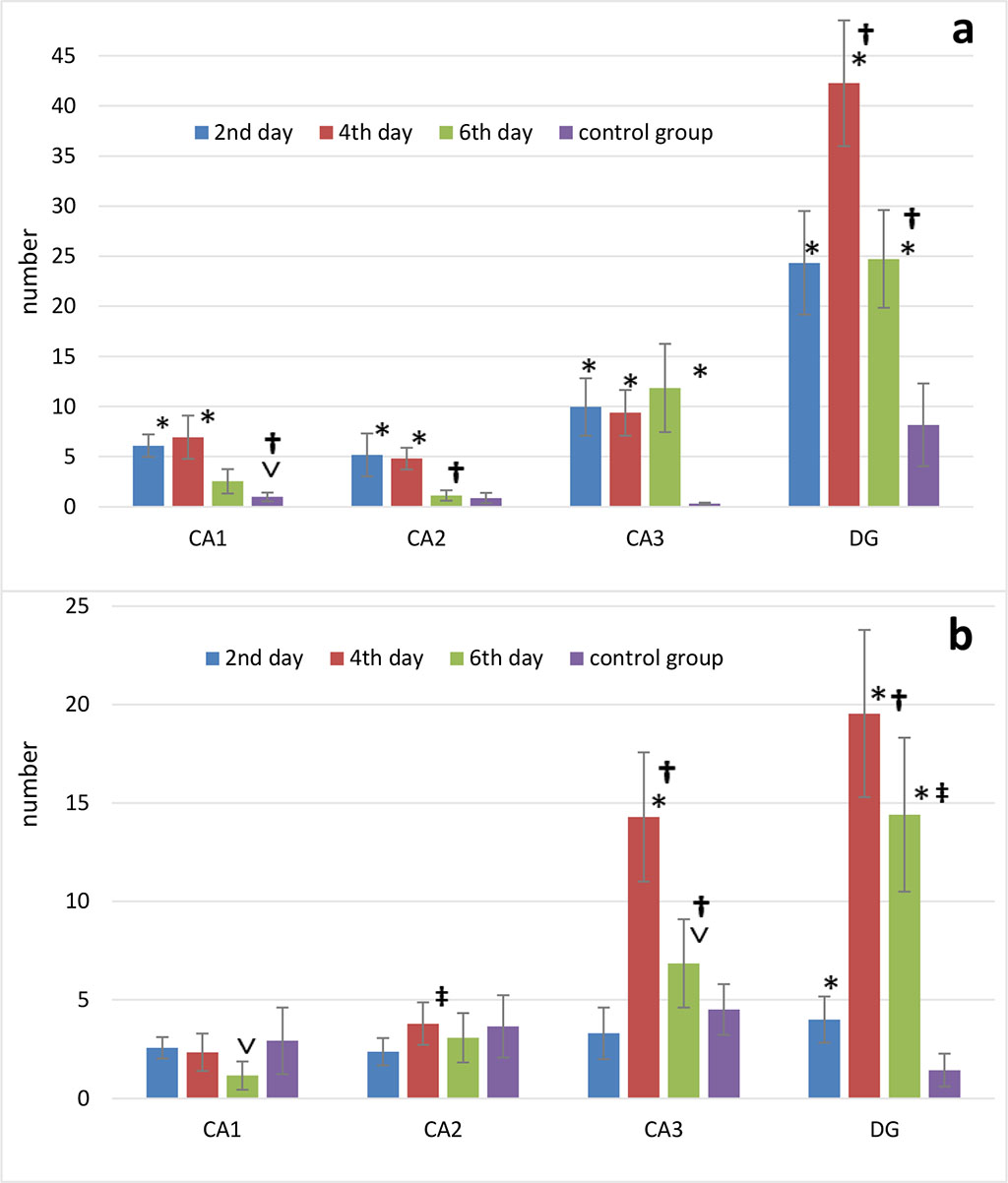
Figure 3. Changes in the number of p53-positive neurons (p53) (a) and dark neurons (b) in the septoplasty simulation. Note: * - significant differences between data of the control group and terms after operation (p<0.001); ˅ - significant differences between data of the control group and terms after operation (p<0.05); † - significant differences between terms after operation within experimental group (p<0.001); ‡ - significant differences between terms after operation within the experimental group (p<0.05).
According to Mann-Whitney test the number of p53-positive neurons in CA1 significantly increased on days 2, 4 (p<0.001) and 6 (p<0.05) after septoplasty compared to control group. Dynamically, the peak of increase in the p53 protein expression in the cytoplasm of CA1 and CA2 neurons of hippocampus occurred on days 2-4, and on day 6 the number of these neurons significantly decreased (p<0.001). On day 6, p53-positive neurons in CA2 did not differ from the control group (Fig.3a). In CA3 there was an increase in p53 protein expression at all time points after surgery compared to the controls (p<0.001).
In DG, compared to the controls, the number of p53-positive neurons was significantly higher at all time points of evaluation. The number of these cells peaked on day 4, compared to the other terms (p<0.001) (Figure 3a).
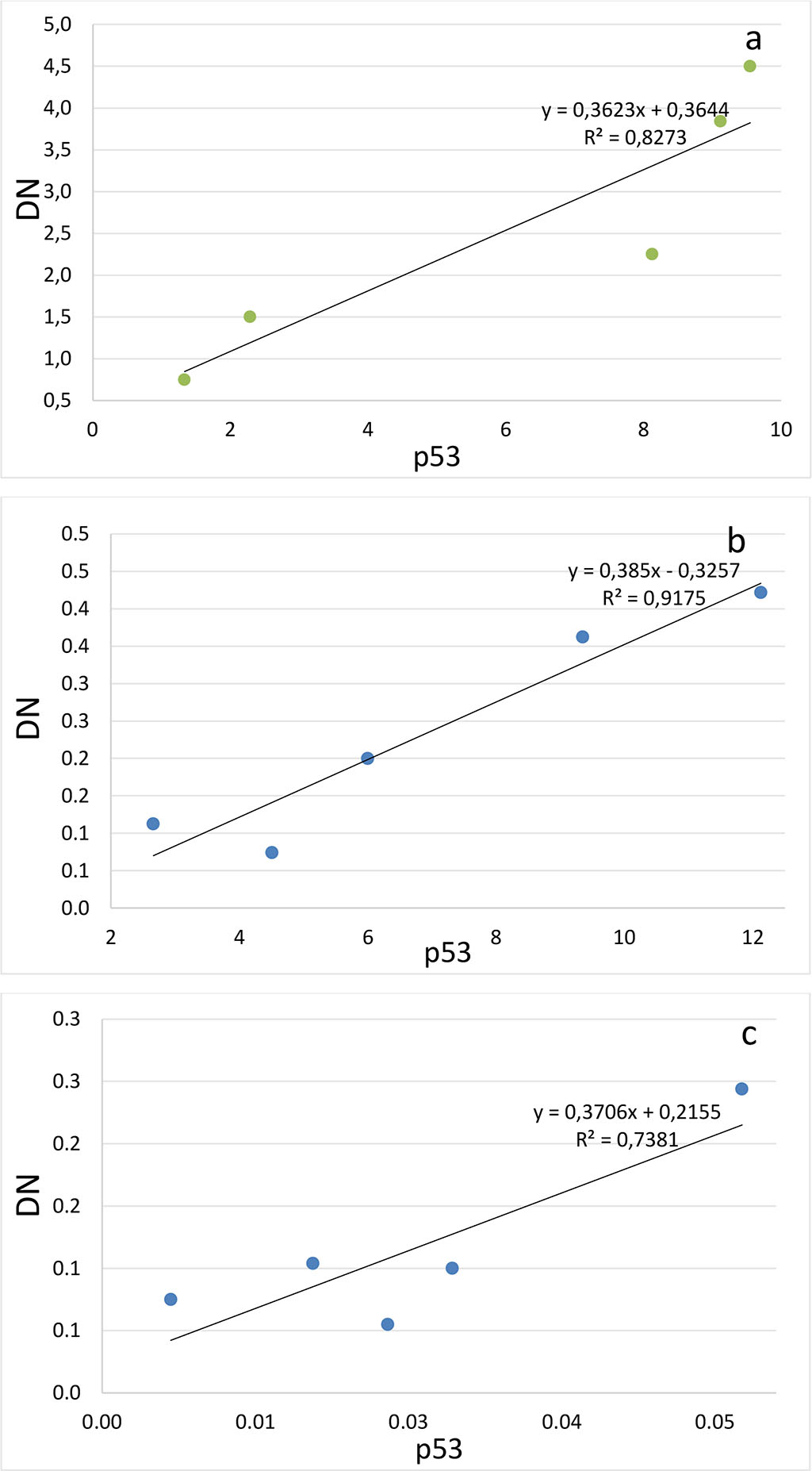
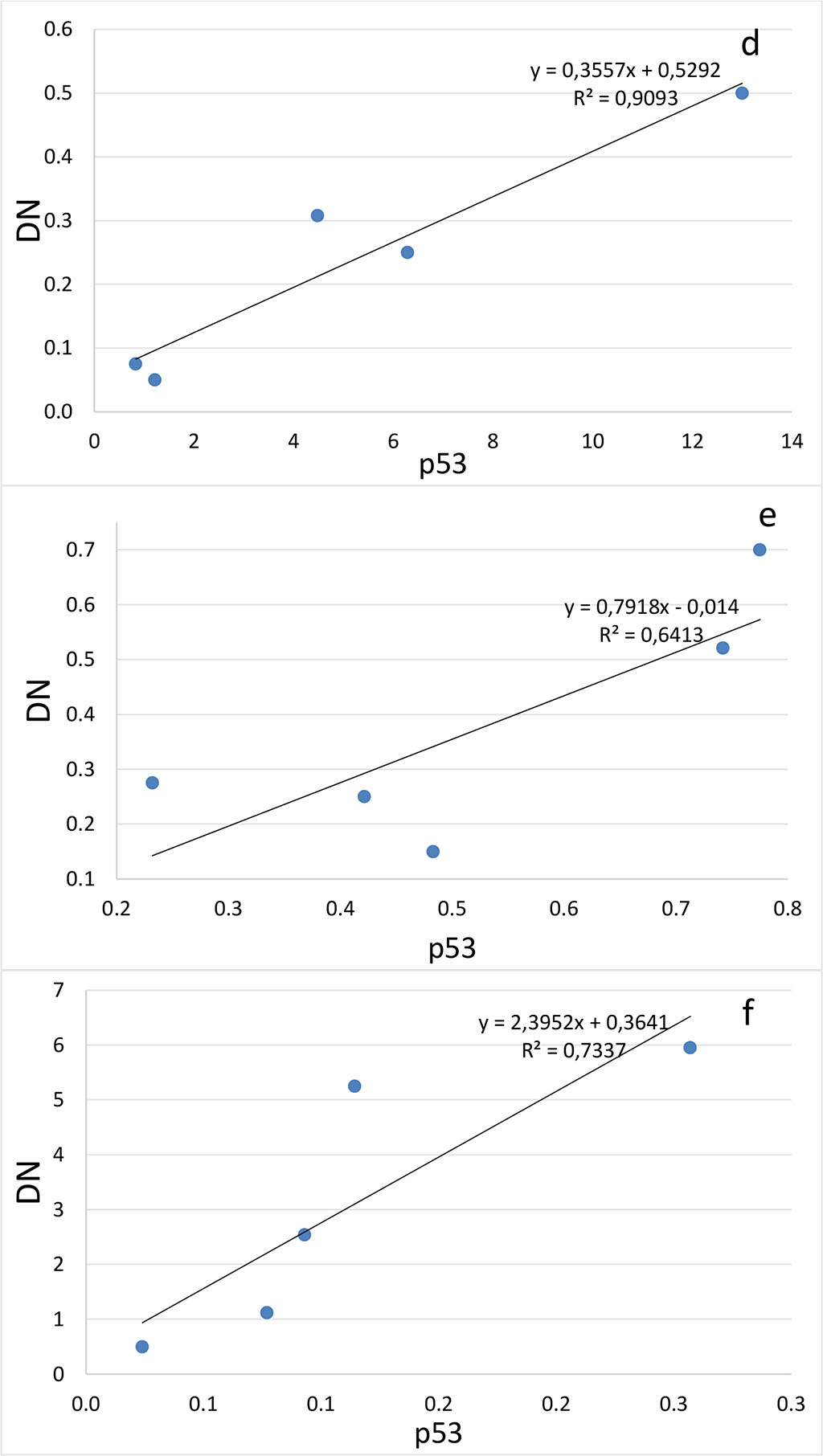
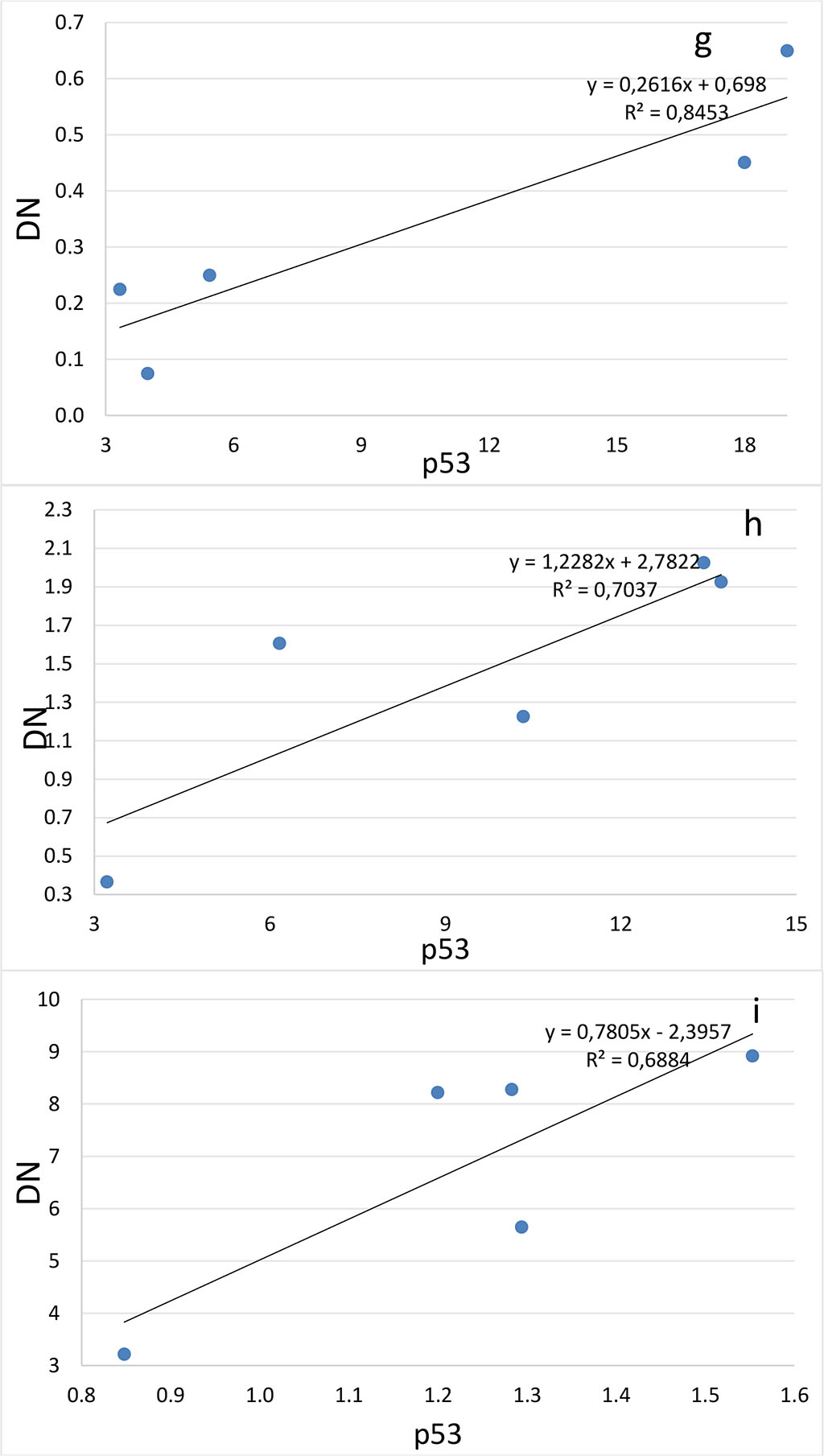
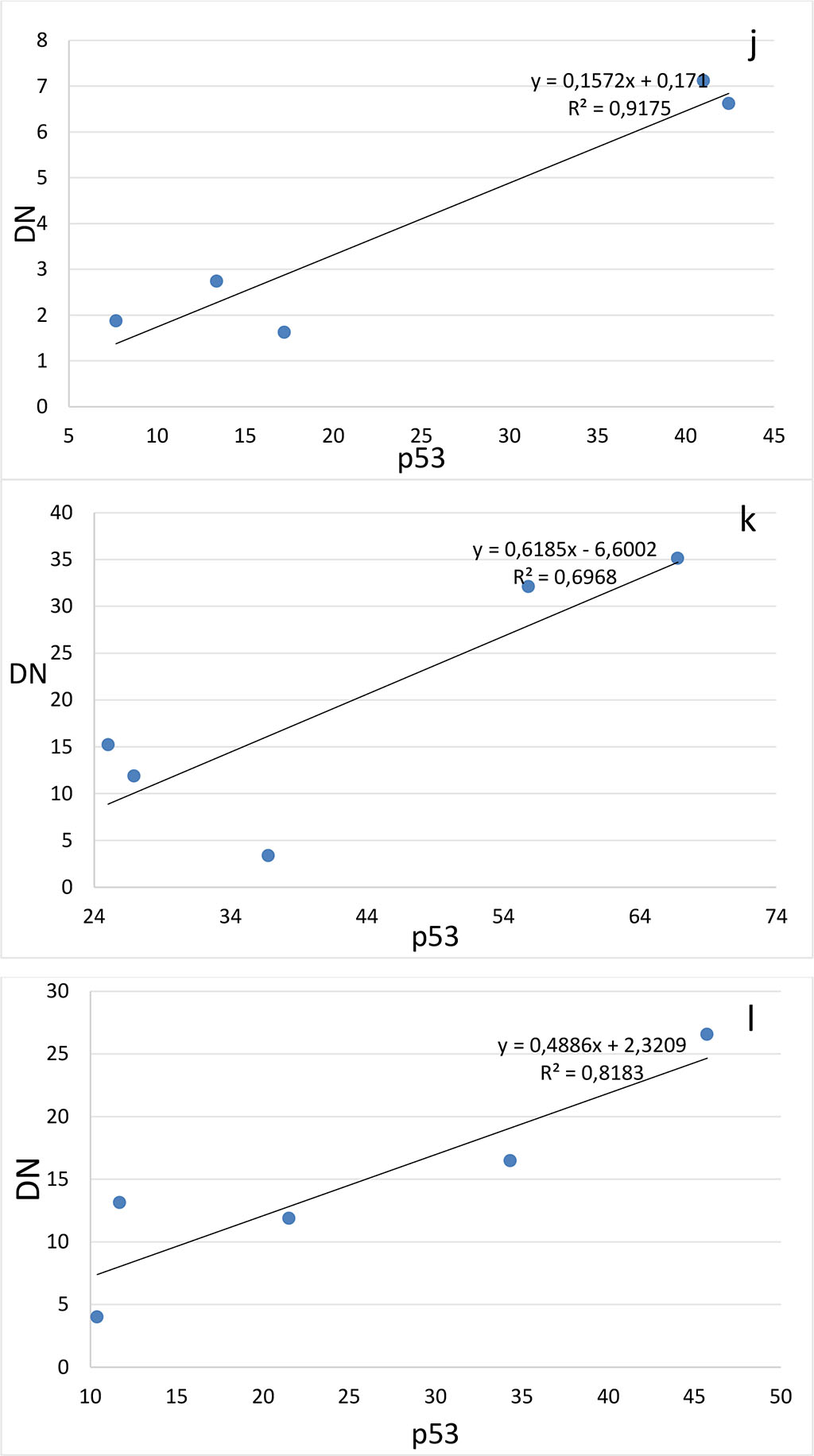
Figure 4. Correlation between the number of dark neurons (DN) and the number of p53-positive neurons (p53) in the hippocampal subfields CA1 (a-c), CA2 (d-f), CA3 (g-i) and DG (j-l) on the 2nd (a, d, g, j), 4th (b, e, h, k) and 6th (c, f, i, l) days after the septoplasty simulation.
Comparing the number of neurons in which the p53 protein was expressed into the cytoplasm and the DN number, a positive strong correlation was found at all evaluation lines and in all hippocampal subfields (Fig.4). The lowest coefficient of determination was found when evaluating the CA2 subfield on the 4th day after surgery (Table 1).
Table 1. Determination coefficients for comparing the number of dark neurons (TN) and the number of p53-positive neurons (p53) in the hippocampus after septoplasty simulation.
| Days after Septoplasty Simulation | |||
| 2-d (R2) | 4-th (R2) | 6-th (R2) | |
| СА1 | 0,80 | 0,94 | 0,71 |
| СА2 | 0,92 | 0,60 | 0,73 |
| СА3 | 0,88 | 0,70 | 0,61 |
| DG | 0,92 | 0,71 | 0,82 |
The p53 protein is activated by cellular stress and DNA damage and, depending on the severity of stress and the specific cell type, can contribute to adaptive responses to stress or can trigger cell cycle arrest or its apoptosis [10]. When normal proliferating cells are DNA damaged, they may react in one of the two ways: cell cycle arrest r apoptosis, and p53 is engaged in both of those processes [11].
The p53 protein is an important component in the neuron apoptosis, for example, after ischemic event or excitotoxicity [12]. An increase in number of such neurons has been demonstrated in ischemia, traumatic brain injuries [13]. Some research studies have proved that p53 is part of the biochemical processes in the cell caused by the activation of NMDA- receptors (N-methyl-D-aspartate) and finally resulting to apoptosis [14].
The mechanisms of proapoptotic action are assumed to be realized through the induction of the TP53 gene (protein p53) expression, the regulation of which inhibits the passage of the cell cycle from G1 to S-phase, which blocks the division of cancer cells and tumor growth [15]. Except to the widely studied role of p53 as a regulator of apoptosis triggering, its neuroprotective role has also been demonstrated [16]. The main goal of neuroprotection is to prevent the death of neurons in the ischemic area, where apoptosis is one of the mechanisms of neuronal death. Bioenergetic processes are slowed down in the penumbra and neurons which have not died yet, remaining in them. In this regard, the absence of neurons in the hippocampus with obvious morphological signs of apoptosis in the analysis of the slices obtained by us, may indicate the presence of neuroprotective properties of the p53 protein. It has been shown that posttranslational modifications of p53 can contribute to the differentiation of neurons, as well as to the growth and regeneration of axons [17].
It was shown that p53 is a neuroprotector in an in vivo model of taupathy[18]. By analyzing the chromatin immunoprecipitation chip, it was determined, that p53 controls the transcription of a group of genes participated in providing synaptic function. Genetic manipulation of these genes changed the neurotoxicity of the tau- protein. The authors have found, that both in mice’s neurons and in the human brain, transcriptional control of these synaptic genes is maintained due to p53. Thus, it has been suggested, that the provision of synaptic function, as a manifestation of neuroprotection, can be performed by p53 protein[18].
In addition to activating the TP53 gene, NMDA receptors participate in caspase-dependent apoptosis, increasing the level of calcium ions, boosting the enzyme caspase-3 activity. This enzyme, in its turn, starts the formation of dark neurons and their next degeneration. When inhibiting caspases with the pancaspase inhibitor FK011, a decrease in the changes characteristic of dark neurons has been achieved [19].
Previously, it was also shown, that dark neurons can both restore their morpho-functional state by increasing the cisterns of the granular endoplasmic reticulum with the formation of membrane curls, the transition of this process to astrocytic processes and, as a consequence, with a subsequent decline in the degree of structural compaction of the cell [7], and be a sign of the final necrotic decay of the cell regardless of the cause of neuron death, including various biochemical cascades of apoptosis [8]. There is an opinion, that dark neurons- are the result of oxidative stress. Thus, it has been shown, that the use of luteolin after brain injury in vivo reduces the number of dark neurons and oxidative stress in them in the hippocampus [20]. It has been shown, that the presence of regenerating dark neurons in the case of animal studies indicates the vulnerability of neuroprotective properties of neuroglia [21]. The presence of such phenomena, as cytoplasmic shrinkage and surface reduction in DN is compared by some authors with the manifestation of neuroplasticity characteristics [22,23]. In fact, plasticity refers to the unique ability of the brain (neuron) to change and reorganize in response to changes in the environment. This property of neurons contributes to their viability and, consequently, the organism’s survival [24].The most well-known examples of neuronal plasticity are the formation of new synapses, the proliferation of dendritic spines, the retraction and simplification of dendrites, and the reduction of dendritic spines under stressful conditions. Some studies have shown that endogenous or exogenous stressors are associated with a decrease in the surface and dendritic spike of neurons [25].
The high determination coefficients found in this study confirm the theory that presence of dark neurons in the hippocampus and dentate gyrus is most likely closely related to the expression of the p53 protein during surgical stress caused by septoplasty simulation in rats. This is probably due to the activation of NMDS receptors in neurons under the influence of surgical stress, as it has been shown that stress leads to an increase in the content of NMDA receptors in the dendritic spike apparatus[26]. In addition, exposure to a large amount of glutamate leads to functional changes in neurons and subsequent launch of the apoptosis program [27]. Stress is known to result in degeneration of hippocampal neuron dendrites [28]. In dark neurons, the dendrites are poorly developed or practically absent as a result of modulation of NMDA receptors. This was shown by the example of CA3 subfield neurons in the hippocampus [29]. In addition, chronic stress has been reported to cause atrophy of the pyramidal layer of the subfield CA1 [30, 31], decrease the long-term potentiation of neurons of the hippocampal CA1 subfield [32] and cause apoptosis of neurons, as well as a decrease in the density of dendritic spikes in neurons in the CA1 region of the hippocampus [33]. Thus, it can be assumed that there is a common mechanism that leads to the start of two processes, discussed in this article – the expression of the p53 protein in the cytoplasm and the formation of dark neurons. The trigger of these pathways is probably the activation of NMDA receptors of neurons.
Morphological changes in the hippocamp are confirmed by our previous studies, which showed that septoplasty simulation in rats provokes the development of many stress reactions [34] and even a breakdown in adaptation [35]: an increase in the number of dark neurons [36], the expression of the p53 protein [3], a significant release of corticosterone into the blood plasma[37], and an increase in degranulation of mast cells[38], disturbances in the balance of the autonomic nervous system[39,40], changes in behavior when testing rats in an open field[9], the appearance of anxiety and a depressive-like state[41].
Septoplasty simulation in rat’s results in the pathogenetic cascades onset which consequently changes the morpho-functional properties of neurons of the pyramidal layer of the hippocampus and contributes to their neuroplasticity. Activation of NMDS receptors of neurons during stress, apparently, initiates two ways of neuron life - the beginning of p53 protein expression and the formation of dark neurons. Both ways can finally lead to apoptosis. The formation of dark neurons and the expression of the p53 protein in them are most likely to be interconnected and can probably provide neuroprotective mechanisms.
The authors declare no conflicts of interest
The keeping of rats, modeling of surgical trauma – septoplasty, as well as the removal of animals from experience were carried out in accordance with the ethical standards established by the Geneva Convention "International Guiding principles for Biomedical Research Involving Animals" (Geneva, 1990).