- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.3
The purpose of the research was to study the features of the expression of growth mediators and matrix metalloproteinases in patients with lower limb varicose veins, clinical classes C4-C6. The study included 98 patients. The control group consisted of 102 healthy donors. The levels of VEGF, PDGF, matrix metalloproteinases (MMP-2, MMP-9), superoxide dismutase, and prolidase were studied. The average value of VEGF in pathologically altered vein (PAV) wall samples was 40.1 ± 6.2 ng/g of protein and was statistically significantly higher - 1.7 times compared with the same value in persons from CG 2 (23.0 ± 5.9 ng/g protein, p=0.001). Patients with lower limb varicose veins (LLVV) showed an inverse correlation between VEGF levels and SOD activity in blood plasma and PAV (r=-0.52; p=0.002 and r=-0.59; p=0.001, respectively). An increase in the level of PDGF correlated with an increase in the oxidative stress index (OSI) in the blood plasma and the PAV wall (r=0.59; p=0.022 and r=0.61; p<0.001). In patients with LLVV, a high level of MMP-9 inversely correlated with the total oxidant capacity (TAC) in blood plasma and in the varicose vein wall (r=-0.48; p<0.001 and r=-0.52; p=0.012). In patients with LLVV, there were statistically significant relationships between prolidase activity and OSI and TAC values in the PAV wall (r=0.62; p=0.003 and r=-0.59; p<0.001). It has been established that VEGF, PDGF, MMPs play a key role in the LLVV pathogenesis; the dynamics of these indicators can be used to predict the effectiveness of surgical treatment and the risk of recurrence.
Keywords: lower limb varicose veins, VEGF, PDGF, matrix metalloproteinases (MMP-2, MPP-9), superoxide dismutase, prolidase, oxidative stress
It is assumed that the excessive production of reactive oxygen species (ROS), accompanied by their accumulation, triggers a cascade of biochemical processes - intensifies oxidative modification of lipids, proteins and nucleic acids, which causes dysfunction and can provoke programmed cell death [1,2,3]. In turn, the activation of lipid peroxidation (LPO), the formation of modified lipoproteins and augmentation of their level in macrophages play a significant role in the progression of endothelial dysfunction [4,5]. Endothelial dysfunction, in turn, playing an inspiring role in the cascade of successive reactions occurring in the venous wall, leads to its morphological inversion and is a key pathophysiological link in the development and progression of lower limb varicose veins (LLVV) [6]. Accordingly, the degree of endothelial dysfunction has been shown to be related to the clinical severity of the disease [7]. Moreover, according to Ortega, M.A. et al. [8], endothelial dysfunction is a key pathophysiological link between the excessive production of ATP and LLVV, which confirms its importance in the progression of inflammation and the development of the disease. At the molecular level, ROS-induced expression of key genes of vascular endothelial growth factor (VEGF) is activated and LLVV progresses [9]. A number of studies have demonstrated an increased expression of VEGF in the walls of pathologically altered vein (PAV) in patients with LLVV compared with controls [10]. Traditionally, this growth mediator is considered a key stimulator of angiogenesis and cell proliferation, which makes it possible to consider it as a potential marker of LLVV. VEGF producers are macrophages, fibroblasts, lymphocytes, polymorphonuclear cells, osteoblasts, endothelial cells, SMCs (smooth muscle cells), mesangial cells of the renal glomerulus, platelets and keratinocytes [11,12,13]. It is well known that VEGF plays an important role in the formation of vasa vasorum [14]. The molecular effects of VEGF include the intensified degradation of the extracellular matrix, processes of migration, proliferation and adhesion of endothelial cells, the formation of vascular structures, the coordination of vascular wall permeability, the initiation of the production of serine proteases, and the inhibition of MMPs (matrix metalloproteinase) expression [15]. Increased expression of VEGF leads to intensification of immune adhesion, recruitment to PAV, and indicates a high risk of progression of LLVV. An experimental study by Fernández-Robredo P. et al. [16] demonstrated the potential role of the imbalance of the oxidant-antioxidant system in the intensification of the expression of this growth mediator. Perhaps, a similar situation occurs in patients with C4-C6 LLVV. This issue needs further study.
Purpose: To study the features of the expression of growth mediators and matrix metalloproteinases in patients with lower limb varicose veins, clinical classes C4-C6.
The study included 98 patients with C4-C6 lower limb varicose veins. Control group 1 (CG1) consisted of 96 patients who had undergone coronary artery bypass grafting. Control group 2 (CG2) consisted of 102 absolutely healthy people.
Evaluation of the expression of growth mediators (VEGF, PDGF (platelet growth factor, matrix metalloproteinases MMP-2 and MMP-9, as well as prolidase)) at the local level was studied by sandwich enzyme immunoassay using ready-made commercial reagent kits. The activity of the superoxide dismutase (SOD) enzyme in erythrocytes was determined by the method of V.A. Kostyuk, based on the reaction of oxidation of quartzetin using test kits from Randox Laboratories (UK).
Statistical analysis of the obtained data was carried out using the STATISTICA 8.0 software package (StatSoft. Inc., USA). The compliance of the values of normal distribution indicators was checked using the Kolmogorov-Smirnov method. Comparative analysis of differences in quantitative indicators between groups was carried out using the Mann-Whitney U-test with a non-parametric distribution and using Student's t-test with a normal distribution. The critical level of statistical significance (p) when testing hypotheses was considered to be <0.05.
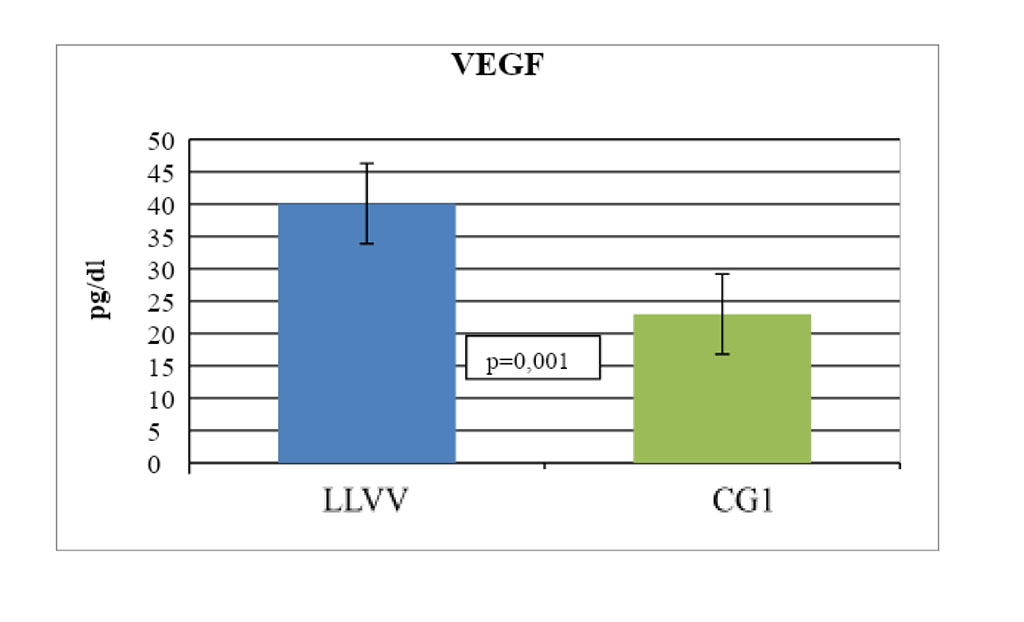
We have revealed changes in the expression of growth mediators and MMPs in patients with C4-C6 LLVV compared with the CG2. The average value of VEGF in the PAV wall samples was 40.1 ± 6.2 ng/g of protein and was statistically significantly higher - 1.7 times compared with the same indicator in individuals from CG 2 (23.0 ± 5.9 ng/g protein, p=0.001) (Fig. 1). At the same time, in patients with LLVV, we noted an inverse correlation between the level of VEGF and SOD activity in blood plasma and PAV (r=-0.52; p=0.002 and r=-0.59; p=0.001, respectively).
Figure 1. Statistically significant changes in the expression of vascular endothelial growth factor in the wall sample of pathologically altered great saphenous vein in patients with C4-C6 LLVV
The average PDGF in the PAV wall samples was 0.17 ± 0.03 ng/mg and was statistically significantly higher - 2.8 times compared with the same indicator in CG1 individuals (0.06 ± 0.01 ng/mg, p =0.040). An increase in the level of PDGF correlated with an increase in the oxidative stress index (OSI) in the blood plasma and the PAV wall (r=0.59; p=0.022 and r=0.61; p<0.001).
The activity of MMP-2 and MMP-9 was recorded both in the samples of the PAV wall and in the control veins. At the same time, the average value of MMP-2 in the PAV wall samples was 25.0 ± 9.0 OD units/mg of solute and was statistically significantly higher - 2.2 times compared with the same indicator in the CG 1 (11.3 ± 4.7 OD units/mg of solute, p=0.022).
The average value of MMP-9 in the PAV wall samples was 23.9±10.6 OD units/mg of solute and was statistically significantly higher - 2.4 times compared with the same indicator in the CG 1 (10.0±3, 5 OD units/mg of solute, p=0.012). In patients with LLVV, a high level of MMP-9 inversely correlated with the total oxidant capacity (TAC) in the blood plasma and the PAV wall (r=-0.48; p<0.001 and r=-0.52; p=0.012).
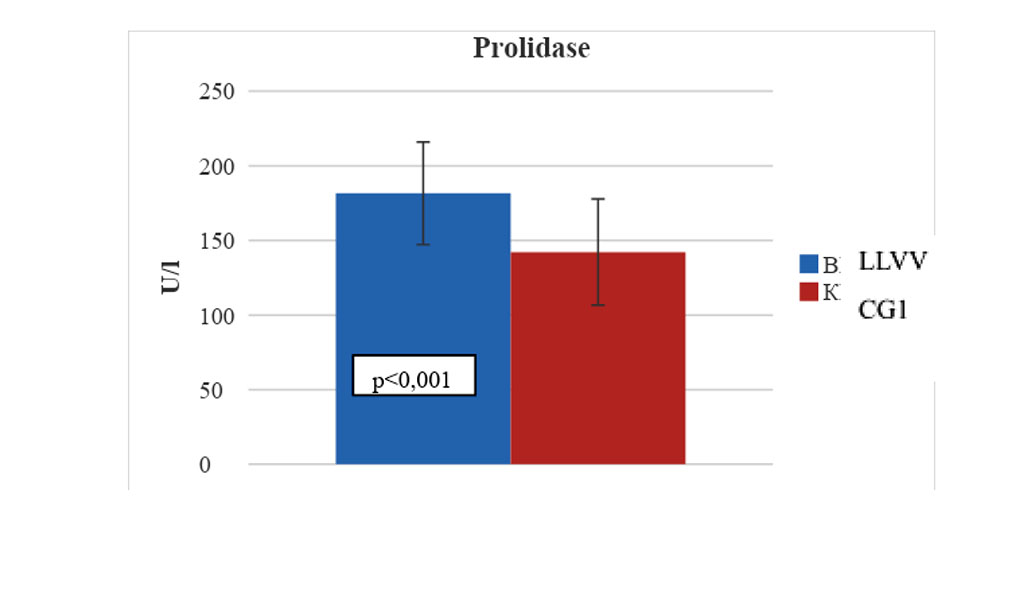
The average indicator of prolidase activity in the PAV wall samples was 181.6 ± 34.4 U/l, respectively, and was statistically significantly higher - 1.3 times compared with same indicators in CG1 (142.2 ± 35.6 U/l , p<0.001) (Fig. 2). In patients with LLVV, there were statistically significant relationships between prolidase activity and OSI and TAC values in the PAV wall (r=0.62; p=0.003 and r=-0.59; p<0.001).
Figure 2. Statistical changes in the prolidase activity in the wall sample of pathologically altered great saphenous vein in patients with C4-C6 LLVV
The results of our multivariate logistic regression analysis (standardized by sex and age profile) of the association of growth mediators and MMPs with the risk of developing C4-C6 LLVV are presented in Table.
Table 1. The results of a logistic regression analysis of the relationship between the indicators of growth mediators and metalloproteinases and the risk of developing C4 - C6 LLVV
| Indicator | В | Multivariate analysis (system level) | р | |
| OR | 95% CI | |||
| VEGF | 0,752 | 2,776 | 2,368 – 3,691 | 0,003 |
| PDGF | 5,248 | 2,308 | 1,772 – 2,689 | 0,006 |
| ММР-2 | 0,643 | 0,834 | 0,611 – 1,138 | 0,254 |
| ММР-9 | 1,537 | 2,241 | 1,964 – 2,634 | 0,002 |
| Prolidase | 0,833 | 0,873 | 0,671– 1,157 | 0,174 |
Note: B - beta coefficient, OR - odds ratio, CI - confidence interval, p - significance level.
It has been established that VEGF, PDGF, and MMPs play a key role in the pathogenesis of LLVV; the dynamics of these indicators can be used to predict the effectiveness of surgical treatment and the risk of recurrence.