- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.20
Malignancy in the cervix mucous membrane often develops in the junction zone of the ectocervix to the endocervix, which is the area of transformation of the stratified squamous epithelium into a single-layer cylindrical epithelium. The reasons for the insufficiency of immune protection in the transition zone of the epithelium from multilayer to single-layer cylindrical at the present stage do not have exhaustive explanations.
Employing the method of immune histochemistry for the localization of immunocompetent cells identification was established by the dynamics of quantitative changes in CD-positive cells pool in the structure of cervix mucous membrane of women in the postmenopausal period from 1 to 5 years. A decrease in the number of CD-positive cells in all parts of the cervical mucous membrane was established in studied phenotypes: CD4, CD8, CD68, and CD163. Differences in the immune representation in the structure of the mucous membrane of the exocervix, the place of transition of the exocervix to the endocervix and endocervix were established. The reasons for the differences in the barrier protection of the epithelium, the authors associate with a greater microbial load of the exocervix, as well as relative immunodeficiency in comparison with other parts of the mucous membrane of the cervix, and an imbalance in the system for ensuring the plasticity of keratocytes in the transition zone of the exocervix to the endocervix with necrohormones and caylons.
Keywords: cervical mucosa, postmenopause, malignancy, immunocytes, phagocytes, proliferation, immune homeostasis, epithelial barrier.
The question of the reasons for the greatest vulnerability to pathogens and malignancy in the structures of the cervical mucosa (CCM) in the transition zone of the exocervix to the endocervix is the subject of heated discussions [3, 5, 10]. Some authors attribute the decrease of barrier functions of the epithelium in the transformation zone to the peculiarities of regenerative processes and the complexity of the process of keratinocyte restitution [14]. However, data on the distinctive properties of the barrier properties at the border of the epithelium, associated with the loading of pathogens in the exocervix and the "sterility" of the immunodeficient endocervix, are disputed at the present stage [1, 7].
In particular, the presence of immunocytes and microbial contamination in the upper layers of the epithelium was shown not only on the surface of the endocervix but also in the lumen of the uterine glands [2, 12, 13]. This indicates that the immunological barrier for ascending microbes is functioning poorly in the transformation zone, which also confirms their role as an entry gate for human immunodeficiency virus contamination [11]. More than 90% of cases of malignancy are recorded precisely in the transformation zone of the epithelial plate, which requires clarification of the real immune security and the reasons for more frequent susceptibility to infectious diseases and malignant tumors in this area of the cervical mucosa (CM) in the postmenopausal period [4, 15, 16]. Despite the growth of patients with CM pathology in the postmenopausal period, the mechanisms of aging and the dependence of the damage degree to various areas of the cervix require in-depth study [18].
Aim of the study.To optimize the diagnosis of cervical pathology in postmenopausal women based on the creation of comprehensive clinical and morphological characteristics of the immune protection of CM.
Tasks of research. 1. To characterize the immune protection of the сcervical mucosa in postmenopausal women under conditions of physiological involution of the reproductive system.
2. To characterize the effector CD4/CD8, CD68, and CD163 phenotypes of immunocytes/macrophages in the cervical mucosa of normal postmenopausal women.
We studied 149 biopsy samples of the cervical mucosa of women in Primorsky Krai obtained during hysterectomy for a benign and non-inflammatory cause from postmenopausal women in the ecto-, endocervix and at the transition site of the ectocervix into the endocervix in accordance with the provisions of the Declaration of Helsinki (2000, 2013) and with the permission of the Ethical Committee of the Far Eastern Federal University. Immunohistochemical methods for detecting CD-positive immunocytes/macrophages were performed according to standard DAKO protocols. The study of histological sections was carried out using an Olympus BX52 microscope with original software for morphometry. The number of expressed cells was counted per 100 cells in each section. The illustrative material was obtained using a digital camera with the original DPx25 software.
The number of immunocompetent cells expressed by markers in postmenopausal women is reduced in the zone of epithelial transformation and is especially significant in the area of the endocervix (Figure 1).
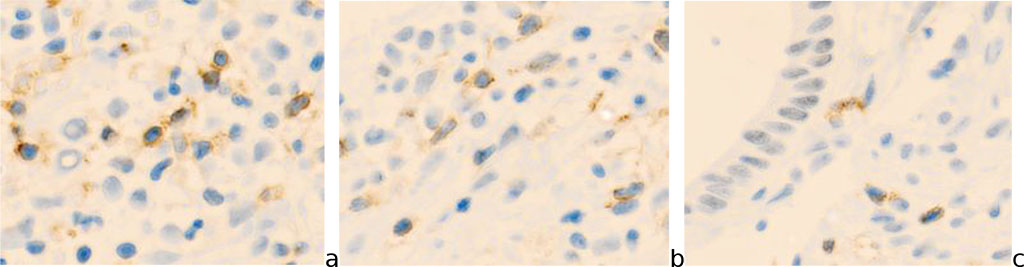
Fig.1- Cervix mucosa of a 54-year-old woman. A) exocervix (CD8); b) transformation zone (junction) (CD4); c) endocervix (CD8). Immune histochemistry to detect the localization of CD4 and CD8 with additional staining with hematoxylin. Microphoto. Zoom x 200.
The number of CD68 effector cells decreases by almost two times in the transformation zone (p<0.01) and is significantly reduced in the endocervix to 1-2 expressed cells per field of vision (Figure 2).
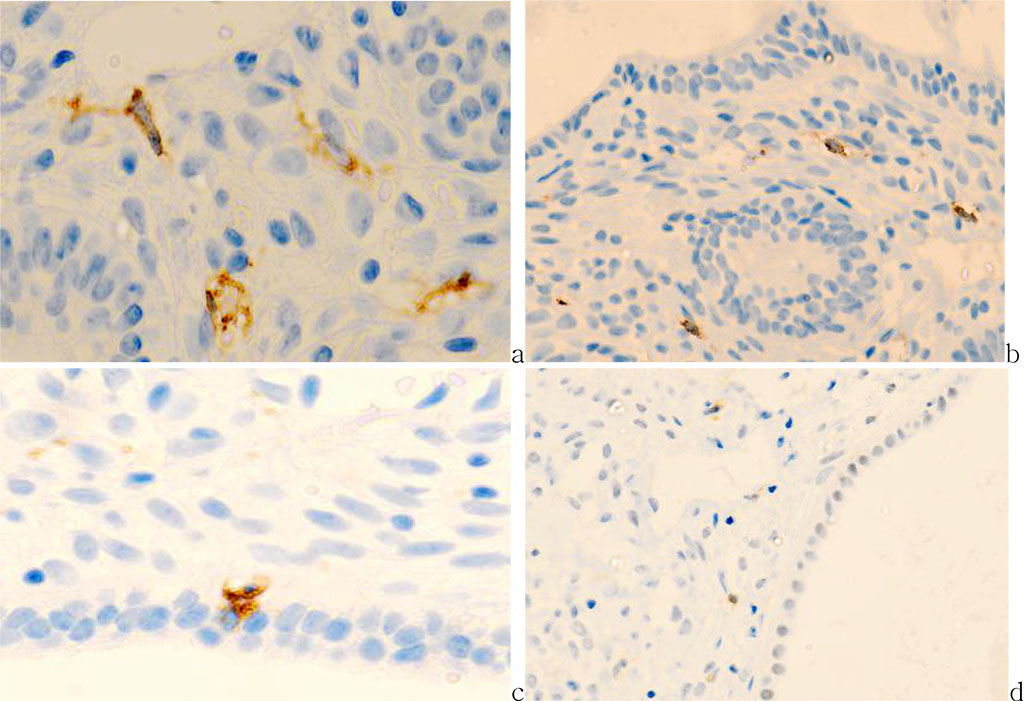
Fig.2 - Cervix mucosa of 56 years old patient. a) exocervix (CD8); b) transformation zone (CD8); c) endocervix (CD68); d) CD163.Immune histochemistry to detect localization of CD4, CD8, CD68 with prestaining haematoxylin-eosin. Microphoto. Zoom a, c) x400; b, d) x 200.
Quantitative data on the content of immunocompetent cells in the structure of SCMS against the background of the postmenopausal period are presented in tables 1, 2 and shown in the diagram in figures 3.
Tables 1. Baseline parameters of immunocompetent cells in cervix mucosa (CM) in the postmenopausal period (per 100 cells in the field of view) in normal course
| Section of CM | Exocervix | Junction | Endocervix |
| CD4 | 15,6+0,31 | 12,8+0,12 | 8,3+0,20 |
| CD8 | 4,5+0,11 | 2,1+0.04 | 1,2+0,05 |
| CD68 | 18,3+2,7 | 16,5+3,5 | 12,4+0,15 |
| CD163 | 24,5+0,34 | 20,7+1,13 | 11,43+0,35 |
*P<0,05
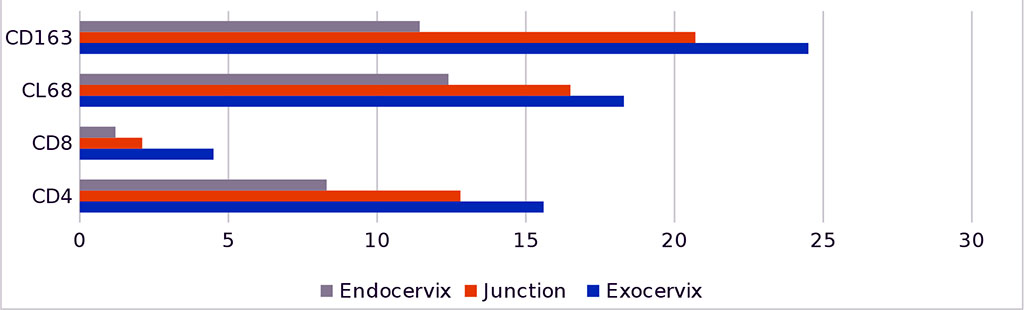
Fig.3 - Dynamics of the distribution of CD-positive cells in the mucous membrane of the cervix
A comparative analysis of the obtained results with the data of other authors showed that T cells (CD3+) are the most common leukocytes in the FRT and are more concentrated in the lower FRT than in the upper FRT [8]. At lower FRT, most T cells are thought to have an effector memory phenotype [17], and 35% of CD45+ mononuclear cells are CD3+ T cells. In addition, immature CD4+ T and CD1a+ DCs are significantly more prevalent in the ectocervix than in the vagina [4].
Contrariwise, CD4+ and CD8+ T cells are equally abundant in the ectocervix, in contrast to the endometrium, where CD8+ T cells predominate [4]. Although NK and B cells are also present in the exocervix, they are much less abundant than T cells [5]. NK and granulocytes (CD66b+) are more numerous in the upper sections of the FRT than in the lower ones [15]. Both T and B cells are more abundant in the exocervix than in the endocervix, which may be related to the degree of higher microbial load in the exocervix [6]. The transformation zone is a sharp transition between immunologically different areas of the exo- and endocervix [16], which may be the reason why exactly in this area is vulnerable to infectious pathogens of a bacterial and viral nature.
Thus, it has been established that CD68 are the most potent antigen-presenting cells and, accordingly, play an important role in the protection of the uterus through the activation of antigen-independent immunocytes, which is necessary for the induction of an adaptive immune response. CD68 were predominantly identified in the basal and suprabasal areas, and their processes reached the basal layers of the stratified squamous epithelium of the exocervix and the transformation zone. CD68-positive cells without typical dendritic processes have also been identified in the intermediate layer. The specific function of these cells remains unexplored; apparently, they are antigen-presenting cells of the intermediate stage of differentiation into specialized ones. The distribution of CD68-positive cells in the subepithelium is uneven, which may be due to the different loading of antigens on the surface of the epithelial layers of the exo-, endocervix, and transformation zone (junction). We noted that the number of CD-positive cells decreases in the direction from the exocervix to the endocervix, where their smallest number is identified.
The quantitative dynamic of CD68-positive cells in the mucous membrane of the cervix is associated with the antigenic load of various departments of cervical cancer.
The exo- and endocervix, as well as the transformation zone of the CM, have differences in the cellular ensembles of immune cell subpopulations.