- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.17
Insufficient supply of iodine in regions with a natural deficiency of the microelement adversely affects the function of the thyroid gland and the state of the dental system in the child population. The aim of this study was to assess the state of the dental status and oral hygiene of the child population living in the territory with biogeochemical iodine deficiency. Our study included 289 schoolchildren aged 12 and 15 years old from three regions of Bashkortostan (Russia) located in the mountains and foothills of the Southern Urals. The following parameters were assessed: concentration of iodine in the urine, content of thyroid-stimulating hormone and free thyroxine in the blood plasma, the state of the mucous membrane and soft tissues of the oral cavity, prevalence of dentoalveolar anomalies, indexes of the CFE/cf and ACI, PAM and SHI.
It has been shown that children and adolescents have a high prevalence of iodine deficiency, accompanied by an increase in the level of thyrotropin in the blood plasma and a decrease in free thyroxine. The schoolchildren of both ages with insufficient iodine supply have a significantly higher frequency of occlusal anomalies, occlusal deformities, anomalies of the dentition and individual teeth; non-carious and carious lesions of the teeth, and periodontal pathology. The children and adolescents with iodine deficiency have worse indices of CFE/cf and ACI, PAM and SHI, and oral hygiene status. The necessity of developing a complex of therapeutic and preventive measures of dental care, combined with an adequate supply of iodine, is stated.
Keywords: iodine deficiency in children, thyrotropin, thyroxin, dental status, oral hygiene.
The territory of the Republic of Bashkortostan (Russia) belongs to the regions with natural iodine deficiency, which causes a constant risk of developing iodine deficiency conditions among the population. Iodine deficiency is most often manifested by hypothyroidism, goiter and its complications, and even cognitive disorders [1,17]. Iodized thyroid hormones are involved in the regulation of the formation of craniofascial structures, the control over the processes of differentiation and proliferation of cells in tissues of the dental system during the antenatal and postnatal periods of development [3,18]. If congenital hypothyroidism is characterized mainly by anomalies of disorders of the dentoalveolar apparatus, then the manifestations of acquired hypothyroidism in children are: periodontal pathology, delayed eruption of milk teeth, the development of carious and non-carious lesions of enamel and dentin, most often hypoplasia of tooth tissues [3,16]. A morphological study of the hard tissues of extracted teeth with a carious process in patients with hypothyroidism revealed a disruption in the shape and location of enamel prisms, a decrease in the density of the dentin structure, deformation and an increase in the diameter of dentinal tubules [5].
An epidemiological study of dental health in the population is necessary to characterize the dental status and determine the population's need for preventive and curative care. Conducting such studies also provides monitoring of the programs used in dental care and enables their correction [14].
Purpose: To characterize the dental status and oral hygiene in the child population living in a region with natural iodine deficiency.
The research was carried out in secondary schools of Beloretsk (Beloretsk region), Karaidel (Karaidel region) and Meleuzovsky (Meleuz region) in the Republic of Bashkortostan, Russia in 2017-2018.
The relief of the Karaidel and Meleuzovsky regions is represented by hills and ridges, single mountains and ridges of the Southern Urals, and the Beloretsky region belongs to the mountainous region of Bashkiria, about a third of its area is located at an altitude of 700 meters above sea level. These areas belong to iodine-deficient biogeochemical territories. 142 schoolchildren aged 12 years and 147 schoolchildren aged 15 years were examined. Informed consent was obtained from the parents or the legal representatives of all children to conduct the study and process personal data.
The clinical examination included an analysis of parameters indicating the general health of the child and local changes. Standard dental examination schemes were used. The red border of the lips, the mucous membrane and soft tissues of the oral cavity, local irritating factors (crowding or other anomalies in the position of the teeth, anomalies in the shape of the dentition and occlusion, areas of premature occlusal contacts, traumatic occlusions, local trauma factors, etc.) were examined, indices were calculated CFE/cf and ACI, PAM and SHI. The examination was carried out under artificial or natural lighting with a standard set of instruments.
Schoolchildren received a single portion of urine and blood samples. Urine was immediately frozen in test tubes at a temperature of -18°--20°C. Determination of the concentration of iodine in urine (CIM) was carried out by the kinetic cerium-arsenite method using commercial reagent kits "Merk" (Germany). Blood was obtained using a BD Vicutainer® vacuum sampling system (USA) containing heparin as a stabilizer. Blood plasma was frozen and stored at -18--20°C.
To characterize the functional state of the thyroid gland in blood plasma, the content of thyroid-stimulating hormone (TSH) and free thyroxine (st4) was determined using VectorBest reagent kits (Russia) by enzyme immunoassay on a Stat Fox 2100 analyzer (USA).
Mathematical analysis of the obtained data was carried out using a professional software package for processing statistical information Statistica 8.0. The type of distribution of the trait for the samples was determined using the Kolmogorov-Smirnov and Shapiro-Wilks test. With a normal distribution of the trait in the sample groups, the levels of the mean and standard deviation (M±σ) were calculated. With asymmetric distribution of the trait, the median (Me) and percentiles of 25% (Q1) and 75% (Q3) were calculated. Differences between samples were assessed using paired Student's t-test or Mann-Whitney U-test. Correlations between traits were calculated by determining Spearman's rank correlation (rs). Differences were considered statistically significant at p≤0.05.
To assess the status of iodine deficiency in the population, the United Nations Children's Fund (UNICEF) and the Global Iodine Network (GIN) recommend studying the median urinary iodine concentration (mURI), since the severity of iodine is highly correlated with iodine intake in the body [12]. There are three degrees of iodine deficiency: mild, moderate and severe. With a mild degree of iodine deficiency, IMF is determined within the range of 50-99 µg/l, with an average degree of iodine deficiency - 20-49 µg/l, a severe degree is characterized by a decrease in IMF less than 20 µg/l. The optimal level of iodine supply varies depending on the age and functional state of the organism from 90 to 290 mcg/l. [1]. The recommended intake of iodine for boys aged 11-14 is 130mcg/day, for girls of the same age - 150mcg/day, for adolescents of both sexes 14-18 years old - 150mcg/day,
The obtained results of ioduria in the children and adolescents in our studies generally indicate a mild degree of iodine deficiency (Table 1). However, it should be noted that 21.8% of young schoolchildren and 19% of adolescents have moderate iodine deficiency, and 12.7% of children and 6.1% of adolescents have severe iodine deficiency.
Table 1. Median and frequency of distribution of iodine concentration in urine among the examined schoolchildren
| Population group | n | Me[Q1-Q3] | Number of samples (abs/%) with urinary iodine concentration (mcg/l) | ||||
| twenty | 20-49 | 50-99 | 100-200 | ˃200 | |||
| Children 12 years old | 142 | 85.4[53.3-118.6] | 18/12.67 | 31/21.83 | 30/21.12 | 48/33.82 | 15/10.56 |
| Teenagers 15 years old | 147 | 99.4[61.3-127.5] | 9/6.12 | 28/19.04 | 22/19.04 | 58/39.46 | 20/13.62 |
p-value
The prevalence of dental anomalies in children aged 12 and 15 years from the areas with iodine deficiency, was 68.3±2.5% (97 children) and 73.46±2.1% (108 children), respectively. The prevalence of dentoalveolar anomalies was considered for various types of anomalies. As a whole, combined dentoalveolar anomalies were detected in 40 children (28.16±2.5%) and 48 children (32.65±2.55%), respectively. At the same time, occlusion anomalies were found in 12 (12.37±1.83%) children in mixed dentition and in 11 (10.18±1.7%) children in the period of permanent dentition. The structure of anomalies of occlusion in the children in mixed dentition and permanent dentition is presented in Table 2. A slightly lower proportion was accounted for anomalies of the dentition, which were detected in 10 (10.3±1.54%) children in mixed dentition and in 9 ( 8.33±1.86%) of children in the period of permanent occlusion. The prevalence of anomalies of individual teeth in the period of removable and permanent dentition was found in 14 (14.43±1.86%) children and in 16 (14.81±1.68%) children, respectively. Non-carious lesions of the teeth in the period of mixed dentition were detected in 23 (23.71±2.06%) children, in the period of permanent dentition in 26 (24.07±2.03%).
The high prevalence of dentoalveolar anomalies in the children with insufficient provision of microelements indicates the negative impact of iodine deficiency on the frequency of their detection.
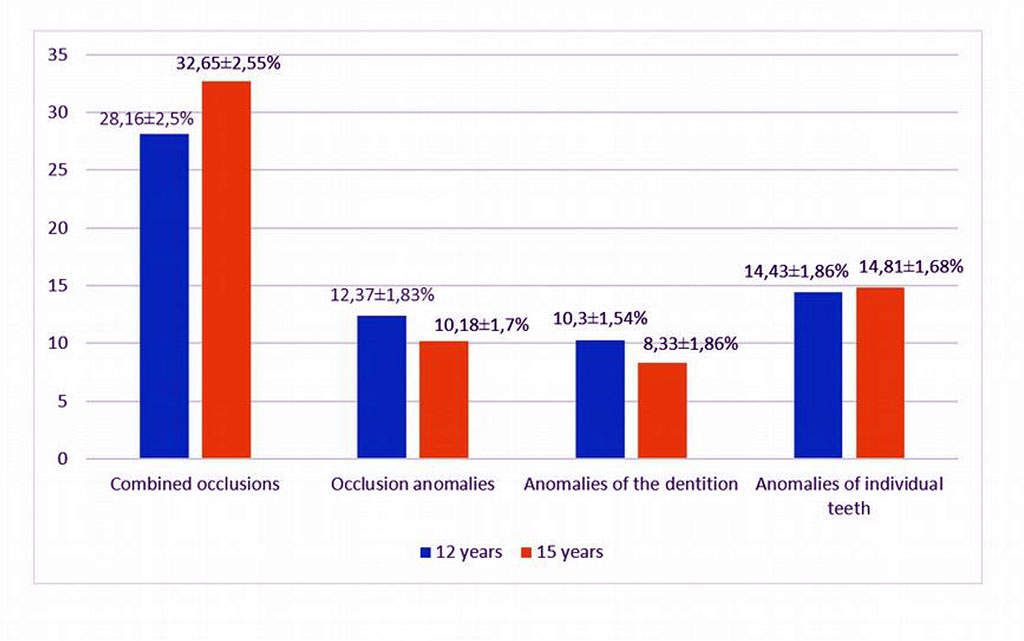
Figure 1. The prevalence of dentoalveolar anomalies (%) in the examined children
Table 2. The structure of anomalies of occlusion in children in mixed dentition
| Disto cclusion | Mesio occlusion | Deep incisal occlusion | Deep incisal disocclusion | vertical incisal disocclusion | cross occlusion | |||||||
| ac. h. | % | ac. h. | % | ac. h. | % | ac. h. | % | ac. h. | % | ac. h. | % | |
| Interchangeable bite (12 years old) | 3 | 25.0 | one | 8.33 | 3 | 25.0 | 2 | 16.7 | 2 | 16.7 | one | 8.33 |
| Permanent occlusion (15 years) | 2 | 18.2 | one | 9.1 | 3 | 27.2 | 2 | 18.2 | 2 | 18.2 | one | 9.1 |
In addition, the results obtained demonstrate that no significant changes in the frequency of their detection have been observed in the region over the past decade. So, according to S.V. Averyanov [2], who studied these indicators among children aged 7–15 years in 2000–2008. in different cities of Bashkiria, the prevalence of dentoalveolar anomalies ranged from 57.9% in Beloretsk to 73.2% in Ufa. A high frequency of detection of anomalies of the dentition among children of the region was also established by other authors [9].
The results of studying the indices of dental status and oral hygiene among the examined schoolchildren indicate their dependence on the degree of iodine supply (Tables 3 and 4).
Table 3. Indices of dental status and oral hygiene in schoolchildren aged 12, (M±σ)
| The degree of iodine deficiency (ioduria, mcg / l) | Floor | n | Prevalence of caries | CPU scores | PEC | UIG | RMA,% | PHP |
| Physiological level (100-150) | AND | 28 | 78.57±2.29 | 3.47±0.339 | 1.15±0.166 | 0.88±0.113 | 31.9±4.23 | 1.03±0.121 |
| M | twenty | 82.14±2.89 | 3.21±0.511 | 1.29±0.238 | 0.78±0.181 | 31.6±5.81 | 1.02±0.171 | |
| Light degree (50-99) | AND | fourteen | 78.89±2.31 | 7.24±3.363 р=0.035 | 1.14±0.198 p=0.062 | 1.01±0.112 р=0.033 | 31.8±2.38 p=0 | 1.08±0.089 р=0.315 |
| M | 16 | 82.75±2.91 | 6.91±2.840 р=0.042 | 1.29±0.161 p=0 | 1.05±0.088 p=0.024 | 37.5±3.78 р<0.001 | 1.28±0.279 р=0.038 | |
| Average degree (20-49) | AND | 16 | 82.63±2.14 | 7.69±2.252 p<0.001 | 1.26±0.103 р=0.018 | 1.03±0.122 р=0.026 | 37.2±3.25 р=0.002 | 1.32±0.099 р=0.0016 |
| M | fifteen | 83.94±2.32 | 7.57±0.534 p<0.001 | 1.59±0.327 р=0.004 | 1.15±0.308 р<0.001 | 37.8±2.16 р<0.001 | 1.35±0.079 р=0.035 | |
| Severe (<20) | AND | ten | 85.86±3.08 | 8.94±3.831 р<0.003 | 1.29±0.402 p=0.384 | 1.11±0.069 р<0.001 | 38.1±1.64 p<0.001 | 1.37±0.092 р<0.001 |
| M | eight | 89.59±3.14 | 9.66±3.297 p<0.001 | 1.68±0.132 р<0.001 | 1.73±0.149 р<0.001 | 43.4±4.24 р<0.001 | 1.58±0.142 р=0.006 | |
| Oversupply (>200) | AND | eight | 86.68±2.98 | 6.87±2.601 р=0.044 | 1.17±0.212 р=0.772 | 0.98±0.229 р=0.836 | 32.6±4.04 р=0.101 | 1.12±0.177 р=0.056 |
| M | 7 | 89.14±3.26 |
6.65±1.480 p=0.037 | 1.39±0.311 р=0.238 | 1.05±0.123 р=0.016 | 36.7±5.36 р=0.041 | 1.19±0.231 р=0.017 |
At the age of 12, the prevalence of caries in permanent teeth in females and males was 78.57±2.29% and 82.14±2.89%, respectively. According to the prevalence of caries in permanent teeth in the age group of 12 years, the study areas can be classified as areas with a “high” prevalence of caries (81-100%) (WHO, Geneva, 1980).
So, in the 12 years old children as the severity of iodine deficiency develops, an increase in the KPU index is observed both among the girls and the boys. The KPU index in children with a physiological level of iodine sufficiency (KIM 100-200 µg/l) is 3.47%±0.339 points in the girls and 3.21±0.511 points in the boys, and in the group with severe iodine deficiency (KIM ˂20 µg /l) - respectively 8.94±3.831 points (Р=0.03) and 9.66±3.297 points (р˂0.001). A similar dynamics of changes is also revealed when determining the caries intensity level index (CIC).
Table 4. Indices of dental status and oral hygiene in schoolchildren aged 15, (M±σ)
| The degree of iodine deficiency (ioduria, mcg / l) | Floor | n | Prevalence of caries | CPU scores | PEC | UIG | RMA,% | PHP |
| Physiological level (100-150) | AND | thirty | 76.66±2.4 | 3.90±0.462 | 1.45±0.057 | 0.94±0.093 | 33.5±3.13 | 0.98±0.096 |
| M | 28 | 82.14±2.62 | 3.55±0.651 | 1.68±0.095 | 1.29±0.074 | 40.8±3.18 | 1.28±0.088 | |
| Light degree (50-99) | AND | 12 | 76.94±2.44 | 4.20±0.773 р=0.173 | 1.51±0.083 р=0.002 | 1.15±0.104 р<0.001 | 33.7±3.22 р=0.832 | 1.11±0.121 р=0.012 |
| M | twenty | 83.08±2.83 | 4.36±1.831 р=0.068 | 1.84±0.096 р<0.004 | 1.36±0.144 p=0.017 | 44.6±3.21 p<0.001 | 1.24±0.124 p=0.556 | |
| Average degree (20-49) | AND | 13 | 85.63±2.92 | 6.16±1.214 p<0.001 | 1.57±0.046 р=0.007 | 1.22±0.134 p=0.018 | 37.1±2.04 р<0.001 | 1.36±0.098 р<0.001 |
| M | fifteen | 88.35±2.89 | 6.53±1.643 p<0.001 | 1.81±0.247 р=0.174 | 1.41±0.119 р=0.002 | 44.5±2.93 р<0.001 | 1.45±0.161 р<0.001 | |
| Severe (<20) | AND | 6 | 86.44±2.87 | 6.83±0.793 р<0.001 | 1.79±0.271 р=0.012 | 1.46±0.124 p<0.001 | 47.3±4.34 p<0.001 | 1.43±0.112 р<0.001 |
| M | 3 | 91.43±2.98 | 8.52±4.053 р=0.048 | 2.04±0.386 р=0.124 | 1.40±0.209 р=0.808 | 53.2±6.02 р=0.471 | 1.48±0.255 р=0.192 | |
| Oversupply (>200) | AND | 12 | 88.46±2.56 | 4.36±0.831 р=0.071 | 1.41±0.047 р=0.014 | 1.16±0.064 p<0.001 | 36.6±3.03 р<0.001 | 1.28±0.123 р=0.018 |
| M | eight | 92.34±2.83 | 4.83±0.743 р=0.024 | 1.72±0.067 р=0.183 | 1.30±0.213 р=0.638 | 37.8±3.49 р=0.057 | 1.23±0.094 p=0.181 |
Correlation analysis data also testify to the relationship between the state of hard tissues of the tooth and the level of iodine supply. Between the concentration of iodine in the urine and the KPU index, the presence of a feedback of medium strength is revealed: rs=-0.57 (p=0.028). In 15 year old schoolchildren, there is also an inverse relationship between KIM and the KPU and UIC indices (rs=-0.51, P=0.038 and rs=-0.44, P=0.046, respectively).
The results of our research state that the prevalence and intensity of damage to permanent teeth in the 12-year-old children and the 15-year-old adolescents in the iodine deficiency regions is higher than in Russia and the Republic of Bashkortostan as a whole, according to the data of an epidemiological survey conducted in 2018. [14].
Definition of papillary marginalalveolar index (C. Parma, 1960) indicates the presence of moderate gingivitis in both age groups of schoolchildren. In the group of 15-year-old girls without iodine deficiency, the PMA index is 33.6±3.13%, in the group of boys it is 40.8±3.18%. In groups with severe iodine deficiency, a higher prevalence of inflammatory disorders of the oral mucosa is found: in girls 47.3±4.39%, in boys 53.2±6.02%. Correlation analysis revealed a negative relationship between the RMA index and the level of KYM. Thus, the Spearman rank correlation coefficient between KIM and RMA was rs=-0.44 (P=0.037) for 12-year-old schoolchildren, rs=-0.53 (P=0.021) for 15-year-old schoolchildren.
The PHP index was used to characterize oral hygiene. As can be seen from the presented data, in children of both age groups with an increase in iodine deficiency, an increase in the index of effectiveness of oral hygiene is found, while they are within the satisfactory range from 0.98 ± 0.096 in girls of 15 years old with a physiological level of iodine sufficiency (table 4) to 1 .58±0.142 in 12-year-old boys with severe iodine deficiency (Table 3). The dependence of the state of oral hygiene on iodine supply is indicated by the results of calculating the rank correlation coefficient: negative relationship between medium and weak strength. The data obtained when determining the GIG index in children do not contradict these results.
Summing up the assessment of the dental status and oral hygiene of the schoolchildren living in an iodine-deficient region, it should be recognized that the lack of iodine supply for children and adolescents has a significant negative impact - with an increase in the severity of iodine deficiency, dental health deteriorates.
The identified indicators of dental status and oral hygiene in schoolchildren with iodine deficiency can be associated with various factors of exogenous and endogenous nature, but the most likely cause is hypothyroidism. Diffuse non-toxic goiter, in conditions of natural iodine deficiency, is often combined with dental diseases [4,10,11,13,16]. After thyroidectomy in experimental animals, decay of the interprism substance in the tooth enamel, a violation of the compactness of the arrangement of prisms, degenerative changes, demineralization and destruction of the enamel with the formation of erosions on its surface are observed [6]. Inhibition of thyroid function during experimental stress leads to atrophy of the alveolar process, increased tooth mobility, reducing the resistance of hard tissues of teeth to cariogenic effects [7,8,15]. In patients with hypothyroidism, compared with individuals without gland pathology, an increase in the number of teeth with destruction of enamel and dentin was observed, the KPU index was 2 times higher, and the number of teeth with non-carious defects was 6 times more [13].
In our studies, in the schoolchildren with iodine deficiency, regular changes in the secretion of thyroid-stimulating hormone and free thyroxine were observed - an increase in the content of TSH in the blood plasma and a decrease in fT4. Table 5 shows the data obtained from a survey of the 15-year-old adolescents. A similar dynamics of deviations in the levels of thyrotropin and free thyroxine was also revealed in the study of the thyroid system in the 12-year-old schoolchildren.
Table 5. The content of thyroid hormones in the blood plasma of 15-year-old schoolchildren in groups with different levels of iodine supply, Me[Q1-Q3].
| Level of iodine supply | n | mKIM mcg/l | TSH, mIU/l | WithТ4, nmol/l |
| Physiological | 58 | 137.5 [117.1-143.3] | 2.32 [1.85-3.40] | 15.3 [14.2-16.1] |
| mild iodine deficiency | 32 | 70.4 [66.2-88.4] | 2.47 [1.90-2.67] | 15.1 [13.6-16.0] |
| The average degree of iodine deficiency | 28 | 34.8 [30.6-45.5] | 3.79 [2.84-4.09] | 14.0 [11.7-15.2] |
| Severe degree of iodine deficiency | 9 | 17.6 [15.2-19.0] | 4.91 [3.68-5.27] | 11.7 [10.1-13.3] |
| Oversupply | twenty | 242.4 [226.5-270.6] | 2.16 [1.52-2.88] | 14.4 [12.2-15.8] |
An analysis of individual results showed that TSH content in 17.7% of 15- year-old schoolchildren exceeded the upper limit of the norm (6.4 mIU / l), including in the subgroup with severe iodine deficiency- in 33.3%, with an average degree - in 21.4%, with a mild degree of iodine deficiency - in 12.5%, indicating the likelihood of developing hypothyroidism. Correlation analysis revealed a negative relationship between the concentration of iodine in the urine and the level of TSH in the blood plasma (rs=-58 P=0.004) and a positive relationship with the content of fT4 (rs=0.52, P=0.012).
Determination of the concentration of iodine in the urine of 289 schoolchildren of secondary schools aged 12 and 15 in three regions of Bashkortostan allows us to conclude that the degree of iodine deficiency in the region is mild. The median urinary iodine concentration in the 12-year-old children is 85.4[53.3-118.6] µg/L, in the 15-year-old adolescents - 99.4 [61.3-127.5] µg/L, the proportion of urine samples with iodine concentrations less than 50 µg/l - 34.5% and 25.16%, respectively. In the schoolchildren with iodine deficiency, the prevalence of dentoalveolar anomalies is much more common than in children living in areas with a physiological level of iodine supply. Insufficient supply of iodine is associated with deterioration in the dental status and oral hygiene: the schoolchildren with iodine deficiency have the worst indicators in KPU and UIK, RMA and RNR indices.