- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.15
Background. Premature ventricular contractions (PVCs) are not mentioned in the list of risk factors of stroke.
Methods. In our investigation, we included 440 patients with PVCs more than 700 per 24 hours. In control group, there were 88 patients with PVCs less than 700 per 24 hours. Within instrumental methods we performed echocardiography (transthoracic or transesophageal), Doppler ultrasound of brachiocephalic arteries, 24-hours electrocardiography (ECG) monitoring, digital sphygmography of common carotid arteries (SG); computer tomography or magnetic resonance imaging of the brain. Laboratory tests were used to identify lipids level, hemoglobin A1c. The patients of the main group were divided into two subgroups up to the characteristics of the revealed PVCs. The 1st subgroup comprised 120 patients with PVCs whose ventricular systole occurred in the phase before the peak of transmitral blood flow in the cardiac cycle, regardless of the location of ectopic foci. In 320 patients with PVCs of the 2nd subgroup the systole of ventricular contraction occurred in the phase after the peak of transmitral blood flow in the cardiac cycle. We observed the patients during 1 year from the onset of the investigation and analyzed the appearance of stroke or transient ischemic attack (TIA).
Results. In most instrumental and laboratory parameters subgroups 1, 2 and control group were identical. The most important differences were in hemodynamic and kinetic parameters in accordance to the quantity of PVCs per 24 hours (700 and more in the main subgroups 1,2) and the moment of the ventricular contraction appearance of the PVCs (before or after the transmitral blood flow peak in cardiac cycle). We revealed the following tendency: the earlier PVCs appeared in cardiac cycle the more an increase in hemodynamic and kinetic parameters was observed. All patients were on the common standard therapy. Despite of that, during 1 year from the beginning of the investigation we remarked the statistically significant different appearance of the stroke and TIA within the groups which was more frequent in the 1 subgroup.
Conclusion. PVCs are an additional risk factor of stroke. The most dangerous type is when the ventricular contraction (PVC) appears before the transmitral blood flow peak in cardiac cycle. Increased hemodynamical parameters during the spread of the 1st post-extrasystolic wave cause an additional mechanical trauma of arterial intima and can be the key moment in atherosclerotic plaques non-stability with further defragmentation, embolism and onset of stroke. In patients with PVCs more than 700 per 24 hours the promising research can be using novel oral anticoagulants to prevent the cardiovascular events in the dosages as it is indicated in atrial fibrillation.
Keywords: cryptogenic stroke, premature ventricular contractions, risk of stroke.
Stroke remains one of the top important medical and social problems all over the world. Despite of preventive measures, new cases of stroke every year is registered in up to 800 thousands Americans as well as it is the common pathology within the European Union countries where every year up to 400 thousands deaths reports happened because of the stroke [1-5]. Within the structure, most of the cases (88%) are ischemic stroke (IS) [6, 7]. The main reasons and risk factors for the stroke are atherosclerotic lesions of brachiocephalic arteries, intra-cardiac sources (left atrium appendage thrombi in atrial fibrillation), diabetes mellitus, dyslipidemia, arterial hypertension and others. Prevention measures, such as lifestyle changing, low-salt and Mediterranean diet, exercise, medication compliance (especially anticoagulant therapy in patients with atrial fibrillation, anti-hypertensive drugs, lipids-lowering drugs) as well as surgical treatment of carotid bifurcation atherosclerosis (operation of carotid endarterectomy, carotid stenting) reduced the number of cases of stroke but unfortunately not significant. Thus, the expected number of strokes in the European Union is 1,5 million to 2025 [1, 8-16].
In the list of traditional risk factors of stroke within arrhythmias there is atrial fibrillation. There are several studies stating that the patients with premature ventricular contractions (PVCs) have more frequent cases of stroke, but these arrhythmias are still not added to the list of stoke risk factors [1, 19].
According to the current guidelines of the American Heart Association and American Stroke Association, the term “cryptogenic stroke” can be used in cases of imaging-confirmed stroke with unknown source, despite thorough instrumental and laboratory investigations (including imaging of brachiocephalic arteries, extended rhythm monitoring, echocardiography (EchoCG), and detection of hemoglobin A1c and lipid profile) [1, 20]. Despite of the preventive and diagnostic measures, up to 45% of patients with IS are diagnosed with cryptogenic stroke. That is why studying the possible additional risk factors and discovering new prevention strategies are the main priorities for cardiologists, neurologists, surgeons and other specialists.
Aim of our investigation is to study the appearance of stroke or transient ischemic attack (TIA) in patients with PVCs.
In our investigation, we observed 440 patients (318 men and 122 women) with PVCs more than 700 per 24 hours. Including criteria were PVCs 700 and more per 24 hours, signed agreement for the participation. Excluding criteria were: atrial fibrillation, heart or aorta aneurisms, verified intra-heart thrombi, heart tumors, mechanical prosthetic valves, dilated cardiomyopathy, hematological diseases associated with hypercoagulation, smoking during 7 years and more, hemodynamically significant atherosclerotic carotid bifurcation stenosis (70% and more). In control group, there were 88 patients with PVCs less than 700 per 24 hours.
Within the instrumental methods, we performed echocardiography (transthoracic or, if it was indicated, transesophageal), Doppler ultrasound of brachiocephalic arteries, 24-hours electrocardiography (ECG) monitoring, digital sphygmography of common carotid arteries (SG); computer tomography or magnetic resonance imaging of the brain. Laboratory analyses obligatory included the lipids level, hemoglobin A1c.
We thoroughly analyzed the data of 24-hours ECG monitoring, including the following parameters: the pacemaker (sinus rhythm or not); heart rate, including circadian; supraventricular ectopic activity; ventricular ectopic activity; blockades; dynamics of the PQ interval; dynamics of the ST segment; dynamics of the QT interval; analysis of heart rate variability.
In Doppler ultrasound, we calculated the linear blood flow velocity and volume flow in common carotid artery during the spread of the regular wave, PVC and 1st post-extrasystolic wave. In common carotid arteries SG, we analyzed the kinetic parameters of arterial wall (speed, acceleration, power, work) also during the spread of the regular wave, PVC and 1st post-extrasystolic wave.
We divided patients of the main group into two subgroups up to the characteristics of the revealed PVCs. So, in the 1st subgroup we included 120 patients with PVCs, the systole of ventricular contraction of which appeared in the phase before the transmitral blood flow peak in cardiac cycle, despite of the ectopic center localization. In the 2nd subgroup there were 320 patients with PVCs, the systole of ventricular contraction of which appeared in the phase after the transmitral blood flow peak in cardiac cycle. The subgroup 1 was less in the number, because this kind of PVC is rare.
We observed the patients during 1 year from the onset of the investigation and analyzed the appearance of stroke or TIA. Additional visits were made in 3, 6 and 12 months from the primary visit. All the patients were on the standard therapy recommended by the current ESC guidelines.
In statistical analyses, for the regular wave, PVC and 1st post-extrasystolic wave, we analyzed Doppler ultrasound parameter (linear blood flow velocity, volume flow); speed, acceleration, power, work parameters were calculated by digital SG of common carotid arteries for all patients. We performed one-way analysis of variance (ANOVA) to compare the mean values of the independent groups for each analyzing parameter to calculate the statistical significance (p≤0,05). To estimate the stroke or TIA during 1 year of investigation, we used Cox analysis. We calculated the indices of a four-field table to establish the relationship between the moment of appearance of ventricular contraction of PVCs in cardiac cycle and the development of stroke or TIA within 1 year.
Patients of both subgroups were identical in age and comorbidities (Table 1).
Table 1. Subgroups 1, 2 and control group.
| Parameter | 1 subgroup N=120 | 2 subgroup N=320 | Control group N=88 | p |
| Men – N (%) | 96 (80) | 222 (69) | 45 (51) | p (1, 2)=0.1534, p (1, c)=0.0001, p (2, c)=0.0154 |
| Women – N (%) | 24 (20) | 98 (31) | 43 (49) | |
| Mean age, y.o., M±σ | 63,1±5,3 | 62,8±6,1 | 61,9±5,9 | p<0.05 |
| Smoking less than 7 years | 29 (24) | 72 (23) | 18 (20) | p (1, 2)=0.9960, p (1, c)=0.9702, p (2, c)=0.9941 |
| Family history in cardiovascular diseases – N (%) | 64 (53) | 146 (46) | 40 (45) | p (1, 2)=0.6017, p (1, c)=0.7962, p (2, c)=1.0000 |
| Arterial hypertension 1 grade | 43 (36) | 128 (40) | 37 (42) | p (1, 2)=0.9315, p (1, c)=0.8941, p (2, c)=0.9969 |
| Arterial hypertension 2 grade | 74 (62) | 192 (60) | 45 (51) | p (1, 2)=0.9978, p (1, c)=0.5545, p (2, c)=0.5688 |
| History of carotid endarterectomy– N (%) | 0 (0) | 0 (0) | 0 (0) | NaN |
| Obesity – N (%) | 27 (23) | 68 (21) | 18 (20) | p (1, 2)=0.9993, p (1, c)=0.9268, p (2, c)=0.9397 |
| COPD mild degree – N (%) | 22 (18) | 66 (21) | 17 (19) | p (1, 2)=0.9838, p (1, c)=0.9998, p (2, c)=0.9989 |
| Chronic kidney disease 1 – N (%) | 10 (8) | 29 (9) | 11 (13) | p (1, 2)=0.9993, p (1, c)=0.8633, p (2, c)=0.8736 |
| Chronic kidney disease 2 – N (%) | 40 (33) | 20 (6) | 5 (6) | p (1, 2)<0.0001, p (1, c)<0.0001, p (2, c)=0.9997 |
| Heart failure NYHA I – N (%) | 30 (25) | 83 (26) | 25 (28) | p (1, 2)= 0.9996, p (1, c)= 0.9821, p (2, c)=0.9905 |
| Heart failure NYHA II – N (%) | 90 (75) | 237 (74) | 59 (67) | p (1, 2)=0.9996, p (1, c)=0.7194, p (2, c)=0.8272 |
| History of myocardial infarction – N (%) | 24 (20) | 67 (21) | 17 (19) | p (1, 2)=0.9996, p (1, c)=0.9821, p (2, c)=0.9819 |
| History of stroke or TIA – N (%) | 13 (11) | 34 (11) | 6 (7) | p (1, 2)=1.0000, p (1, c)=0.8598, p (2, c)=0.8261 |
| History of arterial embolism of lower extremities – N (%) | 1 (1) | 3 (1) | 1 (1) | p (1, 2)=1.0000, p (1, c)=0.9995, p (2, c)=0.9998 |
Both subgroups were identical also in lipids level (Table 2).
Table 2. Lipids level in subgroups 1, 2 and control group.
| Parameter/subgroup | 1 subgroup N=120 | 2 subgroup N=320 | Control group N=88 | p | |
| Cholesterol, mmol/l – N (%) | <5 | 85 (71) | 219 (68) | 64 (73) | >0,05 |
| ≥5 | 35 (29) | 101 (32) | 24 (27) | >0,05 | |
| HDL- Cholesterol, mmol/l – N (%) | <1 | 8 (7) | 24 (7) | 3 (3) | >0,05 |
| ≥1 | 112 (93) | 296 (93) | 85 (97) | >0,05 | |
| LDL- Cholesterol, mmol/l – N (%) | <4 | 114 (95) | 307 (96) | 84 (95) | >0,05 |
| ≥4 | 6 (5) | 13 (4) | 4 (5) | >0,05 | |
The data of instrumental investigations also demonstrated that the patients of subgroups 1, 2 were identical (Table 3).
Table 3. Instrumental data in subgroups 1, 2 and control group
| Parameter/subgroup | 1 subgroup N=120 | 2 subgroup N=320 | Control group N=88 | p | |
| Ejection fraction by Sympson, % – N (%) | <45 | 10 (8) | 13 (4) | 0 (0) | p (1, 2)=0.0244, p (1, c)=0.0446, p (2, c)=0.6292 |
| 45-65 | 108 (90) | 289 (90) | 88 (100) | p (1, 2)=1.0000, p (1, c)=0.0195, p (2, c)=0.0205 | |
| >65 | 2 (2) | 18 (6) | 0 (0) | p (1, 2)=0.3894, p (1, c)=0.7434, p (2, c)=0.1539 | |
| Hypokinetic walls in left ventricle – N (%) | 20 (17) | 54 (17) | 0 (0) | p (1, 2)=1.0000, p (1, c)=0.0006, p (2, c)=0.0003 | |
| Ascendance aorta, mm – N (%) | <40 | 120 (100) | 320 (100) | 88 (100) | p=1.0000 |
| Pulmonary artery pressure – N (%) | <30 | 120 (100) | 320 (100) | 88 (100) | p=1.0000 |
| Intra-heart thrombi – N (%) | no | 120 (100) | 320 (100) | 88 (100) | p=1.0000 |
| Coronary arteries stenoses – N (%) | yes | 96 (80) | 263 (82) | 68 (77) | p (1, 2)=0.9847, p (1, c)=0.9897, p (2, c)=0.8358 |
| Hemodynamically insignificant carotid bifurcarion stenoses – N (%) | yes | 42 (35) | 66 (21) | 5 (6) | p (1, 2)=0.0157, p (1, c)<0.0001, p (2, c)=0.0095 |
| Plaque type III – N (%) | yes | 20 (17) | 21 (7) | 1 (1) | p (1, 2)=0.0104, p (1, c)=0.0023, p (2, c)=0.2693 |
| Hemodynamically insignificant renal arteries stenosis – N (%) | yes | 6 (5) | 6 (2) | 2 (2) | p (1, 2)=0.3793, p (1, c)=0.8518, p (2, c)=0.9993 |
The main parameters of arteries hemodynamics differed in all subgroups. So, during the spread of the 1st post-extrasystolic wave, we observed the statistically significant growth of the main hemodynamic parameters in both subgroups, especially in the 1st subgroup (up to 160% in comparison with the regular pulse wave) (table 4).
Table 4. Doppler ultrasound parameters on common carotid artery
| Parameter/subgroup | 1 subgroup N=120* | 2 subgroup N=320* | Control group N=88* |
| Linear blood flow velocity in common carotid artery, m/sec, M±σ | 1,21±0,36** | 0,94±0,22** | 0,72±0,26 |
| Volume flow in common carotid artery, ml/min, M±σ | 576±54** | 432±39** | 360±48 |
| Systolic blood pressure, mm Hg, M±σ | 162±28** | 146±24** | 118±17 |
*p<0,05
**the
data in 1st post-extrasystolic contraction wave
The data of digital SG on common carotid artery repeated the revealed pattern of Doppler ultrasound and also demonstrated the statistically significant growth of main kinetic parameters (speed, acceleration, power, work) during the spread of the 1st post-extrasystolic wave, which was more prominent in subgroup 1. The parameter of work calculated by the digital SG you can see on picture 1.
Figure 1. Main kinetic parameter – work – calculated by the digital SG on common carotid artery. A – regular contraction, B – PVC, C – 1st post-extrasystolic contraction.
Thus, we see that in most instrumental and laboratory parameters subgroups 1, 2 and control group were identical. The most important differences were in hemodynamic and kinetic parameters in accordance to the quantity of PVCs per 24 hours (700 and more in the main subgroups 1, 2) and the moment of the ventricular contraction appearance of the PVCs (before or after the transmitral blood flow peak in cardiac cycle). We revealed the following tendency: if earlier PVCs appeared in cardiac cycle then more the growth of hemodynamic and kinetic parameters was observed.
All patients were on the common standard therapy. Despite of that, during 1 year from the onset of the investigation we remarked the statistically significant different appearance of the stroke and TIA within the groups (Figure 2).
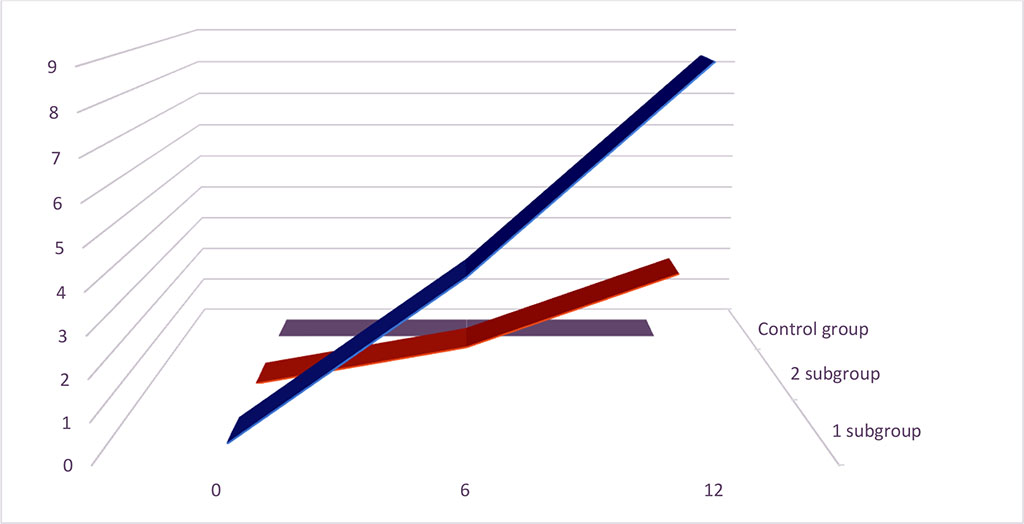
Figure 2. Stroke and TIA appearance (%) during 1 year of investigation.
We calculated the indices of a four-field table to establish the relationship between the moment of ventricular contraction appearance of PVCs in cardiac cycle and the development of stroke or TIA within 1 year. The normalized value of the Pearson coefficient (C`) was 0,316 that demonstrated the average relationship between these two parameters.
Within the heart arrhythmias, it is estimated that atrial fibrillation is the common, recognized risk factor of the stroke and TIA. It is important to underline that within the list of stroke risk factors, there are still no PVCs [1]. Despite of the standard therapy, we observed the appearance of stroke of TIA in 8% patients in subgroup 1 and in 3% patients in subgroup 2. In our investigation, in all cases of stroke the patients had the diagnosis of “cryptogenic stroke”. Moreover, we tried to minimize the influence of traditional risk factors using the excluding criteria for the participation in our investigation. That is why the logical question appears – is there any additional risk factor of TIA and stroke that played the key role in the appearance of complications in these cases?
Several patients of both subgroups had the atherosclerotic plaques of carotid bifurcation area. These plaques were hemodynamically insignificant and patients had no indications for the surgical correction (operation of carotid endarterectomy or carotid stenting). But in consideration of the main hemodynamic and kinetic parameters of common carotid artery growth we believe that these plaques can become the potential source of embolism. When the atherosclerotic plaque had the signs of non-stability (uneven contour, inclusion of calcium etc.) that allows it to be classified as type III, and it comes the increased pulse wave of 1st post-extrasystolic contraction after the long pause, it can become the destructive mechanical force on this plaque. The mechanical trauma can cause an injury of atherosclerotic plaque, its ruptures, parietal thrombosis with further embolism and as the result – appearance of stroke or TIA.
So, we observed the following characteristics of the 1st post-extrasystolic wave:
The maximum parameters we measured in PVCs with ventricular systole occur before the transmitral blood flow peak in cardiac cycle [21, 22].