- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.12
According to the report by the United Nations Office on Drugs and Crime (2020), the number of drug-users aged 12-64 has exceeded 275 million people, which accounts for 5.4% of the globe’s population. The highest number of drug addicts has been registered in the United States of America − 28.6 million; in the European Union – 17.4 million, while Russia accounts for 6 million, with 11.8 million deaths registered annually worldwide, that are directly or indirectly related to drug abuse. Tightening anti-drug measures along with higher demand for drugs from low-income groups of people result in expensive “classical” drugs being replaced with various substitutes, medicines and synthetic drugs, including phencyclidine. Chromatographic methods play a major role in the expert evaluation of narcotic drugs, toxic substances and their metabolic products in biological media, whereas the use of gas and liquid chromatography-mass spectrometry allows identifying – with a high reliability level – most compounds that are relevant in terms of analytical and forensic toxicology. This paper offers a comparative analysis and an overview of optimized methods to be employed to detect phencyclidine and its derivatives in biological fluids and tissues. There has also been toxicokinetics and biotransformation of phencyclidine studied, as well as a drip chemical analysis of phencyclidine carried out. The detection of phencyclidine and its derivatives through chromatographic methods has been improved. The obtained outcomes can be used for differentiating phencyclidine and its derivatives in forensic chemical and chemical-toxicological studies of biological material.
Keywords: narcotic drugs, psychotropic substances, phencyclidine and its derivatives, biological material, gas chromatography with mass-selective detector (GC-MS).
Forensic medicine is a complex system of scientific knowledge, research methods and expert evaluation of facts, based on which medical-biological issues are to be resolved that arise in forensic & investigation practice. One of the major sections within modern forensic medicine is forensic anthropology, which focuses on general, group and individual anatomical and physiological features of human beings in order to identify the personality [2,5,7,9,11,22,25,26], as well as forensic toxicology, which studies the identification and proof of the effects that toxic exogenous substances can have an active physiological effect on the human body [1,3,6,10,14,17,23,30].
Chemical and toxicological studies are important when carrying out a medical examination of drivers in order to identify narcotic intoxication, when monitoring the use of narcotic drugs and psychotropic substances by schoolers, when seeking to detect the cause of a death, as well as during special investigation measures aimed at combating illicit drug and psychotropic substance trafficking [4,12,24,29].
The main issue in detecting narcotic drugs – either natural or synthetic – in biological objects is their low content, which requires highly sensitive research methods. One of such methods is chromatography-mass spectrometry, which relies on a combination of chromatography (gas or liquid) and mass spectrometry. Gas chromatography with mass selective detection is widely used in forensic medicine for screening, whereas the disadvantages of this method include low sensitivity and complex sample preparation procedure. The gas chromatography method with tandem mass spectrometric detection, which offers a higher level of sensitivity and specificity, is employed for screening studies and directional search for substances, the sample preparation still remaining complicated. High-performance liquid chromatography with tandem mass spectrometric detection is the most sensitive method, which requires no complex sample preparation. This method, though, lacks universal libraries of mass spectra [8,18,28].
Phencyclidine (abbrev. Eng – PCP) is a synthetic chemical substance, a derivative of cyclohexylamine, whose molecule contains three different rings – two saturated and one aromatic (Fig. 1). Substances that belong to the phencyclidine type feature structural similarities with phencyclidine and ketamine and are classified as arylcyclo-alkylamines 13.
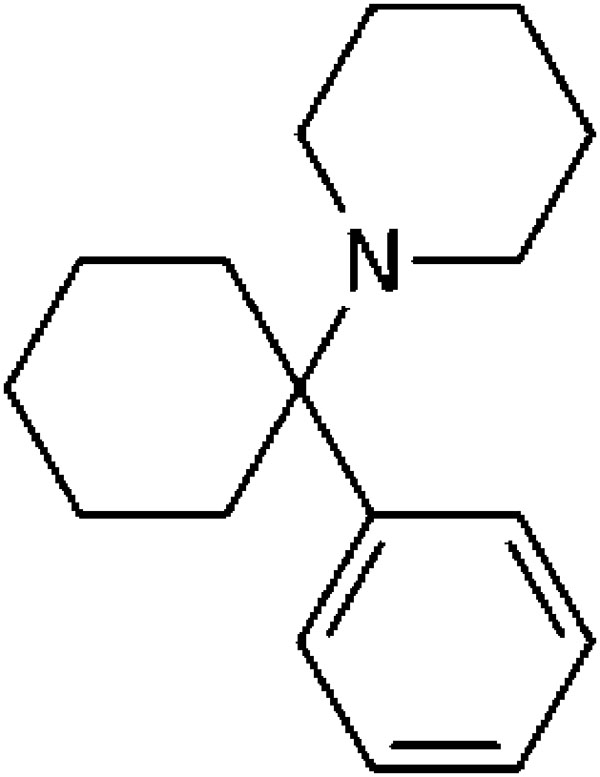
Figure 1. Phencyclidine – 1-(1-phenylcyclohexyl)- piperidine
PCP (Phencyclidine) was synthesized as a pharmacological drug for intravenous anesthesia in the second part of the 1950s in the USA, to be further introduced into medical practice under the name of SERNYL. Unlike opiates, the drug in question inhibits neither cardiovascular activity nor respiration. However, its further clinical use revealed some toxic side effects, including postoperative hallucinations, arousal, mental issues and depressive conditions, and, despite its good therapeutic properties, the drug was taken off clinical practice 16,19. The issues of phencyclidine overdose diagnostics have many difficulties, since the initial symptoms may be similar to schizophrenia manifestation. The overdose symptoms include convulsions, hyperthermia, increased blood pressure, cardiovascular issues, respiratory depression, death from respiratory arrest [15]. The use of phencyclidine can be diagnosed through its detection in such biological fluids as blood (blood serum) and urine. In case a high dose of phencyclidine was used, its high levels in biological fluids may persist for up to 5 days. Given that, the toxicokinetics and biotransformation of phencyclidine in cadaveric biomaterial represents a relevant issue, while the methods of phencyclidine detection require more detailed study.
The aim of this study is to improve chromatography-mass spectrometric research methods and to identify proper sample preparation conditions thus seeking to detect phencyclidine and derivatives thereof in objects of biological origin.
Within this work, the research objects we used were blood, urine, internal organs from dead human bodies (both male and female, aged from 20 to 60 years, with different causes of death), as well as physical evidence (remnants of phencyclidine and its derivatives – ethicyclidine, tenocyclidine, rocyclidine).
When carrying out a forensic chemical examination involving internal organs, the following extraction methods were employed: extraction and sorption when isolating medicinal substances from the biological material while employing the Stas-Otto method, the Vasilieva, Kramarenko, Valov method, the chromatographic study methods [20, 21].
The registration of chromato-mass spectra was carried out employing the Analyst 1.6 software package. The integration of peaks, as well as the calculation of the studied compound quantitative content and the statistical data processing were performed using the MultiQuant 2.4 software.
The choice of the isolation method constitutes an important part of a chemical study focusing on identifying narcotic substances. The most important stage is sample preparation, while improperly selected isolation may result in partial – and sometimes complete – loss of the analyzed substances. When selecting the isolation method, the metabolism of the analyzed drugs is to be taken into account.
After visual selection of the incoming biomaterial, the extraction method was selected, and following the first preliminary steps of the study, all negative samples were dismissed.
The positive samples were used further employing different methods of detecting narcotic substances, with the order of the methods changed.
Depending on the object, the extraction process was divided into solid-liquid type and extraction in a liquid-liquid system. For the solid-liquid system, organic solvents were used, namely chloroform and ether. Both individual (for one substance or group) and general (research on several groups of substances) isolation methods were used through the entire work.
Staining reactions were carried out on porcelain cups with dry residues obtained after extracts evaporation.
Reaction with Marquis agent. The Marquis agent was applied to the dry residue, further observing the color change.
Agents: A1 – 10 drops of 40% formaldehyde solution in 10 ml of glacial acetic acid; A2 – concentrated sulfuric acid.
A small amount of the analyzed sample was placed on the plate, with one drop of A1 agent, and two drops of A2 agent added.
With the Marquis agent, phencyclidine offers weak pink staining.
Reaction with Mandelin agent: phencyclidine was added to a freshly prepared agent (200 ml of sulfuric acid + 1 g of ammonium vanadate), whereas the reaction produced orange staining.
Reaction with Ehrlich agent (1 ml of paradimethylaminobenzaldehyde, 95 ml of 96% ethanol, 20 ml of hydrochloric acid): all this produced red staining with phencyclidine.
Due to constantly progress in analytical toxicology, chromatographic methods go on offering lower detection limits, enhanced screening capacity for the presence of a variety of analytes and accurate detection of analytes in a wide range of complex environmental samples and biological samples [27].
In view of this, a comparative analysis of various solvent systems was performed thus aiming to detect phencyclidine (PCP) and derivatives thereof by thin-layer chromatography.
Kieselgel G F254 was used as the stationary phase, and as far as the mobile phase is concerned, we used Methanol: Ammonia 100: 1.5; Chloroform: Methanol 9:1; Chloroform: Acetone 4:1; Ethylacetate: Methanol: Ammonia (conc.) 85:10:5 and Cyclohexane: Toluene: Diethylamine 75:15: 10.
The developing agents were: agents of Dragendorf, Ehrlich, Marki, iodoplatinate. The agents applied to the chromatographic plates included phencyclidine and its derivatives (ethicyclidine – N-(1-phenylcyclohexyl)-ethylamine; tenocyclidine – 1-(1-(2-thienyl)-cyclohexyl)-piperidine; rocyclidine – 1-(phenylcyclohexyl)-pyrromedin; PCP-1 – piperidinocyclohexanecarbonitrile, a by-product of the PCP prohibited synthesis, which releases cyanide in small quantities during decomposition). The plates were placed in three chambers prepared beforehand:
After the plates were developed, the Rf values were calculated in all the three systems. All the values obtained are shown in Table 1.
Table 1. Rf values in various solvent systems, used for identifying phencyclidine (PCP) and its derivatives by TLC
| Solvent system | Subsrance | Rf х100 |
Ethyl Acetate: Methanol: Ammonia (conc.) 85:10:5 |
Ethicyclidine Tenocyclidine Phencyclidine Rocyclidine |
79 86 84 79 |
Cyclohexane: Toluene: Diethylamine 75:15:10 |
Ethicyclidine Tenocyclidine Phencyclidine Rocyclidine |
65 73 73 66 |
Chloroform: Methanol 90:10 |
Ethicyclidine Tenocyclidine Phencyclidine Rocyclidine |
27 54 35 25 |
An analysis of the data shows that both 1 and 2 solvent systems can be used to detect ethicyclidine, all the three systems are good for tenocyclidine and PCP, yet the second system only is good for PCC (1-piperidinocyclohexanecarbonitrile), since in System 1 the substance did not rise well from the start line (Rf – 0.1), and in System 3 it stayed on the line in question. If there is a need to separate a mixture of these substances, then only one system will not be enough, since in System 1, rocyclidine and ethicyclidine have the same Rf value; with tenocyclidine and phencyclidine, the Rf values in system 1 are very close, which would also make it impossible to separate the two substances. In System 2, tenocyclidine and phencyclidine have similar Rf valued. In System 3, tenocyclidine and phencyclidine are well separated, yet PCC stays at the start point. This means that proving the presence of phencyclidine and its derivatives by thin-layer chromatography will take at least 2 solvent systems to have a reliable result.
There was chromatography-mass spectrometric study performed for phencyclidine and its derivatives.
Gas chromatographer: column DB-5, 30 m (0.25 mm inner diameter; 0.25 microns phase film) or ULTRA 1 (0.2 mm x 0.33 microns); carrier gas – helium. The inlet pressure is appr. 5 psi. The injector temperature is at least 1000 Co above the initial temperature of the column.
The first sample, when analyzing each extraction batch for PCP content, has to be injected into GC/MS with the non-extracted standard registered in the SIM mode in order to identify the retention time.
Detector parameters: helium inlet pressure – ab. 5 psi; detector “ON” − from 1.5 to 2.0 minutes prior to the first peak of interest; detector “OFF” − from 0.5 to 1.0 minutes following the last peak of interest; electronic multiplier voltage – ab. 200 volts above the auto-tuning value.
Ions for SIM analysis: #1 200 PCP; #2 186 PCP; #3 243 PCP; #4 205 d5-PCP; #5 191 d5-PCP; #6 248 d5-PCP.
Table 2 shows the values of characteristic ions in the mass spectrum for phencyclidine and tenocyclidine.
Table 2. Ions in the mass spectrum for phencyclidine and tenocyclidine
| # | Characteristic ion (m/z) | Studied mass spectrum | Phencyclidine | Tenocyclidine |
| 1 | M (molecular ion) | 249 | 243 | 249 |
| 2 | M-29 | 220 | 214 | 220 |
| 3 | M-43 | 206 | 200 | 206 |
| 4 | M-57 | 192 | 186 | 192 |
| 5 | M-77 | - | 166 | - |
| 6 | M-83 | 165 | 158 | 165 |
| 7 | C5 Н5N | 84 | 84 | 84 |
| 8 | C7 Н7 | - | 77 | - |
| 9 | C4Н3S | 83 | - | 83 |
Figures 2 and 3show the mass spectrum of phencyclidine and tenocyclidine.
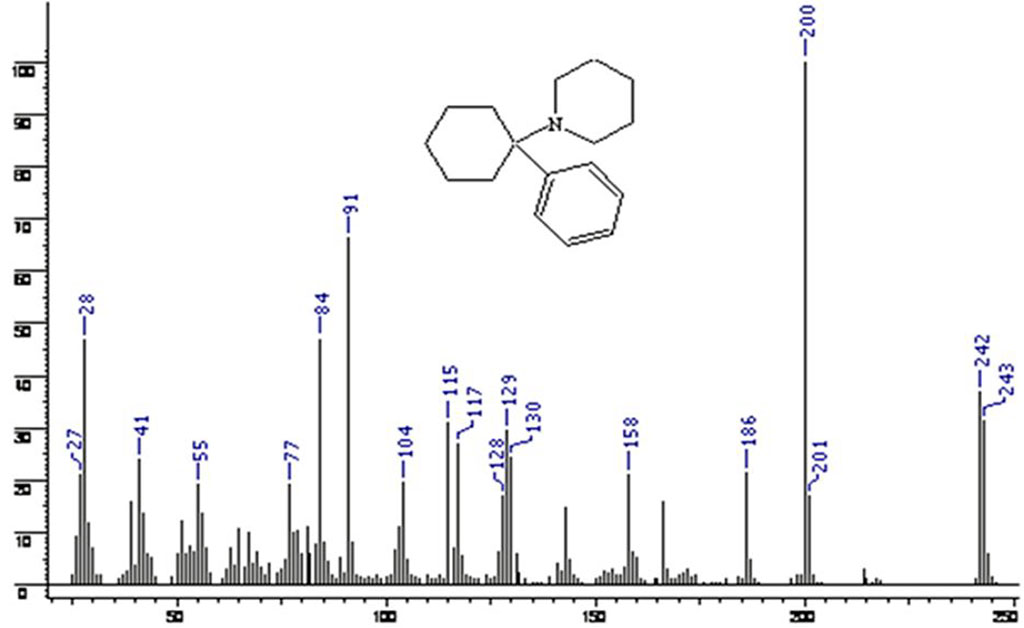
Figure 2. Phencyclidine mass spectrum
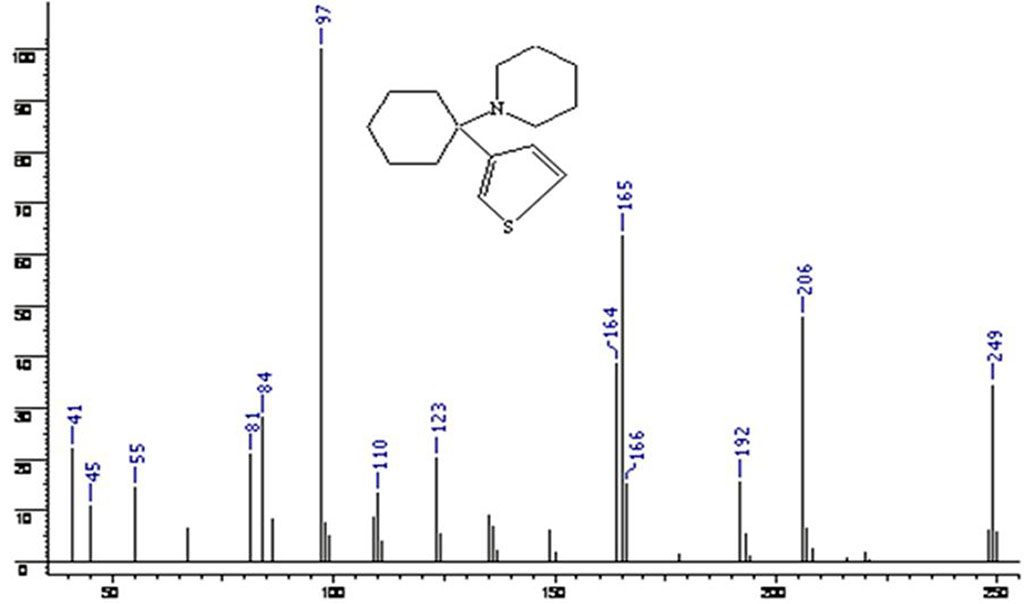
Figure 3. Tenocyclidine mass spectrum