- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 5: e1. DOI 10.35630/2199-885X/2022/12/5.11
We studied the features of acute enteral failure in patients with peritonitis in the setting of COVID-19 by studying the properties of the enteral pathobiome, biochemical, functional, pro-inflammatory and anti-apoptotic markers in diffuse peritonitis. A retrospective randomized cohort controlled study was conducted; it included 84 patients divided into two groups. Stratified randomization was used. The species composition and properties of the enteral luminal pathobiome were studied. 699 inoculations were made, resulting in 3024 isolates. The enteral morphofunctional coefficient, the level of alkaline phosphatase and its intestinal isoform were dynamically studied. In immunohistochemical studies, we studied the expression of CD-3 and Bcl-2. Patients with COVID-19 in addition to a decrease in the level of saprophytic microflora, showed a significant increase in Klebsiella spp., Candida, Clostridium spp. and Citrobacter (p≤0.05). The rate of antibiotic resistance in the comparison group was 8.3%, in the main group - 36.9%. MRSA pathogens were noted in 86.4% of cases, VRSA strains were found in 20.6% of cases. Patients with COVID-19 had a deficiency of intestinal alkaline phosphatase, which persisted even on the day 5 of treatment. Macro- and microscopic studies showed pronounced inflammatory and apoptotic changes in the intestinal wall of patients in the main group. A specific enteral pathobiome that is multiresistant to antibiotics, pronounced inflammatory and apoptotic changes in the intestinal wall, and a predisposition to perforation of the small intestine are characteristics of enteral failure in diffuse peritonitis in the setting of COVID-19.
Keywords: peritonitis, intestinal failure, dysbacteriosis, enteral pathobiome, COVID-19, intestinal alkaline phosphatase, CD-3, Bcl-2
The difficult time of the COVID-19 pandemic, though in addition to many human losses, allowed us to accumulate a lot of clinical experience. Now there is time for summarizing the results, which are based on an analysis of the characteristics of the clinical course of various diseases in the context of the COVID-19 pandemic [1-5].
Patients with COVID-19 often have an enhanced immune response with a hyperinflammatory state characterized by a "cytokine storm" that may reflect changes in microbiota composition. During critical illness, many therapies are prescribed, including antibiotics, sedatives, analgesics, body position, invasive mechanical ventilation, and nutritional support, which can increase the inflammatory response and alter the balance of the patient's microbiota. This state of dysbacteriosis can lead to increased vulnerability of patients and inadequate response to critical circumstances, such as emergency surgery [6-12].
Recently, a large number of studies have appeared on the pathomorphosis of many surgical diseases caused by the COVID-19 pandemic. According to some authors, the endogenous source of infection and the cause of complications is the intestine, especially in abdominal surgery. Some scientists believe that short-term local or generalized intestinal obstruction occurs during any abdominal operation [13-18]. Indeed, the intestinal barrier, consisting of the luminal microbiota, the mucosal layer, and the physical barrier made of epithelial cells and immune cells, plays a special role in health and disease [19-23]. Peritonitis causes intestinal obstruction, first due to inflammation, then due to bacterial toxins, and finally, the resulting fibrinous adhesions can delay the recovery of intestinal function. Bacterial lipopolysaccharide causes intestinal obstruction by initiating an inflammatory response in the smooth muscle layers of the intestine and a subsequent decrease in smooth muscle contractility. One of the protective mechanisms in this case are enzyme systems that inhibit lipopolysaccharides. Intestinal alkaline phosphatase (IAP) – the intestinal brush border enzyme – inhibits inflammatory mediators and is an important positive regulator of intestinal barrier function and microbial homeostasis [24-25].
Purpose of the study: To study the features of acute enteral failure in patients with COVID-19 by studying the properties of the enteral pathobiome, biochemical, functional, pro-inflammatory and anti-apoptotic markers in diffuse peritonitis.
A retrospective randomized cohort controlled study was conducted. In the period 2020-2021 we conducted a comprehensive examination of 42 patients with COVID-19 who developed acute surgical intra-abdominal pathology during the disease, which was complicated by peritonitis and intestinal failure syndrome, requiring emergency surgery. The comparison group consisted of 42 patients comparable in terms of sex, age, as well as the severity and nature of the pathology. Patients in the comparison group had a surgery in 2017-2018 - in the pre-pandemic period. The stratification criteria for inclusion in the study groups were: APACHE II ≥15 points, ACI (V.S. Savelyev's abdominal cavity index) ≥ 14 points. The exclusion criteria were: acute thromboembolic disorder of the mesenteric circulation, stage IV malignant tumors, carcinomatosis, history of antitumor chemotherapy or radiation therapy.
We studied the species composition and properties of enteral luminal microflora obtained from intestinal drainage systems. Analyzer VITEK 2 Compact 30 4700733 (France) was used. Colony-forming units (CFU) were determined in 1 ml of small intestine chyme. Antimicrobial susceptibility testing was performed using the EUCAST disc diffusion method. 699 inoculations were made, resulting in 3024 isolates.
To study the severity of intestinal failure using ultrasonography, the enteral morphofunctional coefficient (EMFC) was dynamically calculated after being determined on days 1, 3 and 5 of treatment. Using the method of enzyme immunoassay by the Cobas e411 device (Switzerland), we determined total alkaline phosphatase (AP) (U/l), its isoform - intestinal alkaline phosphatase (U/l), as well as their ratio of AP / IAP (%) in serum and intestinal contents. The studies were carried out on days 1, 3 and 5.
Morphological studies of the small intestine were performed in case of its resection or at autopsy. Immunohistochemical studies were carried out in 6 patients of the main group and 5 patients of the comparison group. Control data were obtained from intestinal autopsies in patients without acute surgical intra-abdominal pathology (n=12). CD-3 was used as a pro-inflammatory marker and Bcl-2 – as an anti-apoptotic marker. The detection system UltraVision Quanto Detection System HRP Polymer (ThermoFisher, USA) was used.
The method of stratification randomization was used. Statistical relationships between indicators were assessed using the correlation module "Basic Statistics and Tables STATISTICA 10.0". Student's t test was used to determine the significance of p differences between groups. Differences were considered statistically significant at p≤0.05.
In the study of the enteral microbiome in the main group, in the context of a decreased growth of saprophytic microflora (Staphylococcus saprophyticus and Escherichia coli by more than 2 times), we found a significant increase in the presence of Klebsiella spp., Candida, Clostridium spp. and Citrobacter (p≤0.05). It turned out to be characteristic that in the main group there was a significant increase in the Staphylococcus aureus representation (a pathogen that is not a part of the normal enteric microbiome). In the main group, the level of contamination was 1.6x102 CFU per 1 ml, in the comparison group – 6.6x107 CFU per 1 ml (p≤0.05). Fig. 1.
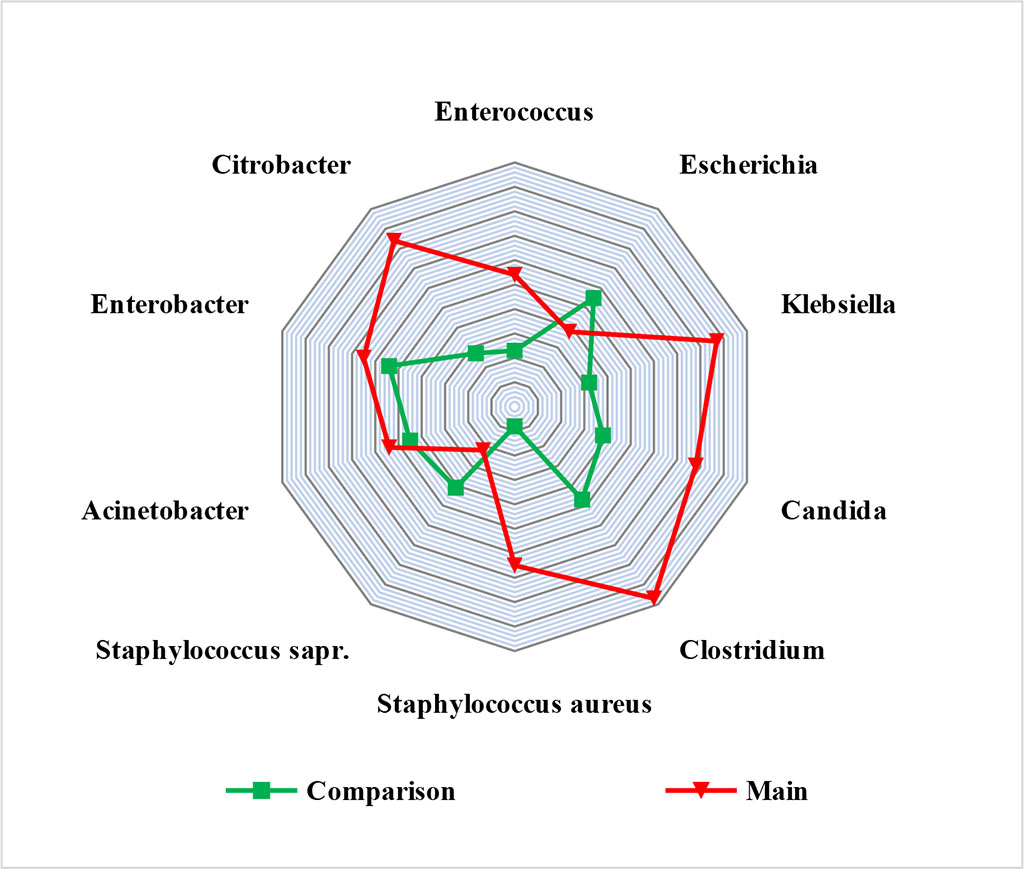
Figure 1. Enteral microbial picture in study groups (CFU×101-10 in 1 ml of small intestine chyme)
Analyzing the data of antibiograms, the data obtained in the main group aroused the greatest interest. An increase in the resistance of the inoculated isolates to all antibacterial drugs was noted. The total rate of antibiotic-resistant strains in the comparison group was 8.3%, in the main group - 36.9%. A high resistance to semi-synthetic penicillins was noted. We found that almost all pathogens were insensitive to ampicillin, and only Enterococcus spp. had an efficiency of 24.4%. Aminoglycosides showed efficacy only against Clostridium spp. (34.3%), Escherichia coli (45.8%) and Citrobacter (18.9%). The greatest sensitivity was found to carbapenems, glycopeptides and oxazolidinones. Staphylococcus aureus was shown to be resistant to almost all antibacterial drugs, with the exception of Linezolid, which was 98% effective. MRSA pathogens were noted in 86.4% of cases, VRSA strains were found in 20.6% of cases.
Functional and biochemical markers of enteral failure at the beginning of treatment in both groups, on average, corresponded to the subcompensated stage and did not differ statistically. In all groups, there was a deficiency of the intestinal isoform of alkaline phosphatase, i.e., there was an imbalance in the ratio of AP/IAP, with the AP predominance, which indicated a pronounced inflammation. On the day 5 of treatment in the control group, the EMFC index was significantly lower (p≤0.05). In the main group, a significant decrease in EMFC was achieved only by the end of the day 5 of treatment. The AP/IAP ratio reliably returned to normal values by the day 5 in the comparison group (p≤0.05). At the same time, there was no recovery of the AP/IAP imbalance in the group of patients with COVID-19. Tab. 1.
Table 1. Changes in biochemical and functional parameters in study groups (M±m; abs.)
| Study groups | Object of study | AP/IAP (%) | EMFC (points) | |||
| Day 1 | Day 5 | Day 1 | Day 3 | Day 5 | ||
| Comparison (n=42) | serum | 0,71±0,21 | 0,33±0,15* | 18,3±0,8 | 5,9±0,3* | 4,5±0,3 |
| chyme | 6,23±0,16 | 2,86±0,09* | ||||
| Main (n=42) | serum | 0,74±0,13 | 0,65±0,11 | 19,4±0,24 | 14,8±1,1 | 10,8±0,6* |
| chyme | 7,01±0,22 | 6,21±0,12 | ||||
* - values at р≤0,05
The macroscopic picture during operations in the main group was characterized by thinning and "sagginess" of the small intestine wall. Visible peristalsis was absent. The serous membrane had bluish areas on it. (Fig. 2) 4 patients of the main group had perforations of acute ulcers in the postoperative period, which were localized in the ileum and required repeated operations. In the comparison group there were no perforations of the small intestine. The morphological picture during intravital studies and autopsies in the main group was characterized by thinning of the mucous membrane, lysis of the cells of the upper part of the villi, while the base of the villi was expanded due to the abundance of lymphoid cells. Paneth cells were absent. There was a massive desquamation of epithelial cells in the area of the tops and side walls with exposure of the connective tissue base. There was a significant increase in CD3+ expression and almost complete absence of Bcl-2+ cells (p≥0.05). Tab. 2.
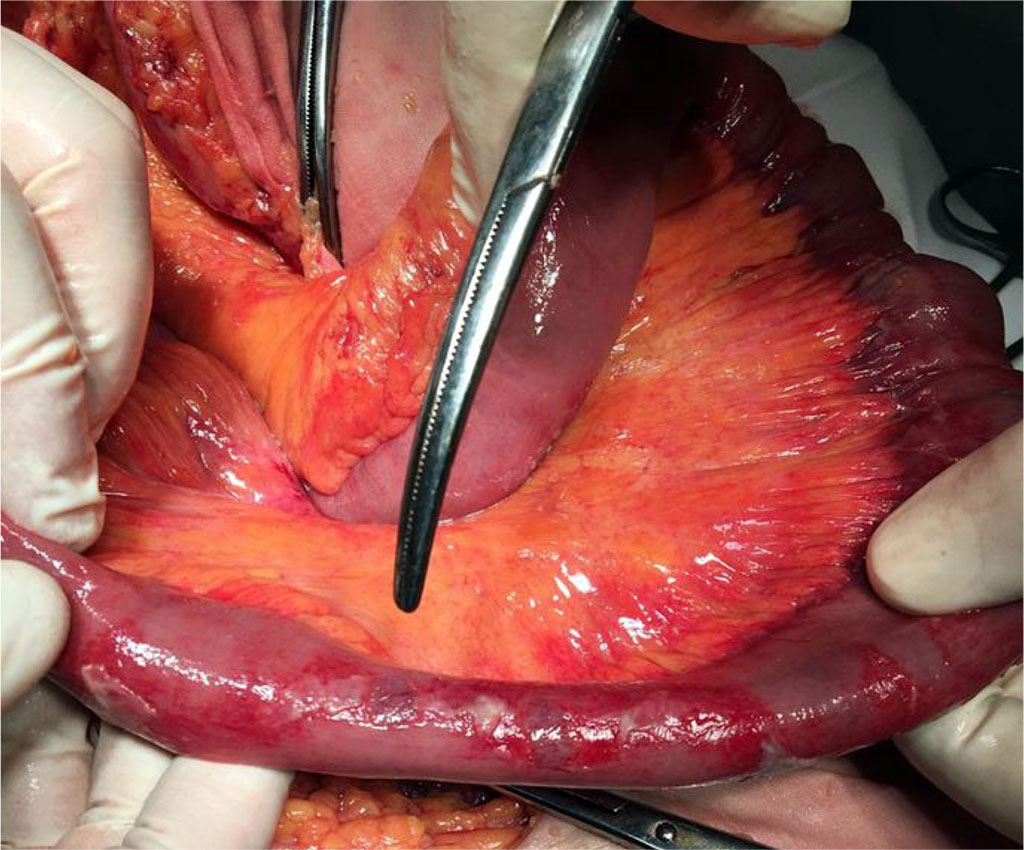
Figure 2. External view of the small intestine with decompensated enteral failure and pre-perforation changes in its wall in patients with peritonitis in the setting of COVID – 19
Table 2. Immunohistochemical parameters of enterocytes in study groups
Parameter |
Control (n=12) | Comparison
group (n=5) |
Main
group (n=6) |
| Me [min; max]; M±sd | |||
| Optical density of enterocytes immunopositive to Bcl-2, c.u. | 0.161±0.033 [0,128;0,193] |
0,
122±0,031 [0,11;0,173] |
0,010±0,005* [0,010;0,026] |
| Relative area of expression of enterocytes immunopositive to Всl-2, % | 12,654±0,345 [7,33;14,23] |
10,987±0,322 [8,32;14,10] |
0,243±0,009* [0,20;0,44] |
| Optical density of enterocytes immunopositive to CD-3, c.u. | 1,03±0,77 [0,67;1,99] |
0,55±0,07 [0,31;0,79] |
2,27±0,44* [1,74;4,23] |
| Relative area of expression of enterocytes immunopositive to CD-3, % | 15,94±2,96 [8,14;22,04] |
8,03±1,12 [5,43;10,06] |
47,04±3,21* [34,21;60,07] |
* - reliability of changes in values at p≤0.05 in relation to the previous study
The formation of the above enteral pathobiome, in our opinion, was greatly impacted by irrational long-term systemic antibiotic prophylaxis and antibiotic therapy carried out before admission to the surgical hospital, as well as drug polypharmacy. Morphological changes in the intestinal wall indicated a high risk of perforation of the small intestine. Rational antibiotic therapy for COVID-19 serves as a preventive measure in the formation of the enteral pathobiome. Restoration of intestinal microcirculation is one of the main pathogenetic methods of therapeutic effect. The high risk of acute perforation of the small intestine calls for special vigilance.
The specific persistent antibiotic-resistant enteral pathobiome, pronounced inflammatory and apoptotic changes in the intestinal wall, and predisposition to intestinal perforations are the characteristics of enteral failure in diffuse peritonitis in the setting of COVID-19.