- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 4. DOI 10.35630/2025/15/4.022
Aims: This review synthesizes current knowledge on inflammatory complications of odontogenic origin, based on scientific literature from 2005 to 2025. The objective is to identify and characterize the full spectrum of complications, their etiology, pathogenesis, diagnostic approaches, and therapeutic strategies.
Methods: A systematic literature search was conducted using PubMed, Scopus, and Web of Science, covering peer-reviewed articles published between 2005 and 2025. The literature search yielded 2,340 articles, of which 28 met inclusion criteria, including 15 original research articles, 10 review articles, and 3 case reports.
Results: The review covers complications including periapical abscesses (periapical, subperiosteal, submucosal), odontogenic sinusitis, osteomyelitis, facial cellulitis, sepsis, orbital abscess, meningitis, cavernous sinus thrombosis, brain abscess, and Lemierre’s syndrome. Contributing factors include untreated caries, periodontal disease, dental trauma, diabetes, and immunosuppression. Infection spread occurs via direct extension, hematogenous dissemination, or lymphatic routes, affecting areas such as the maxillary sinus, orbit, mediastinum, and intracranial spaces.
Conclusions: The results emphasize that early diagnosis, targeted antimicrobial therapy, surgical intervention, and patient education are critical for effective management. The discussion underscores the need for interdisciplinary approaches and preventive measures to mitigate these complications.
Keywords: odontogenic infections, periapical abscess, odontogenic sinusitis, osteomyelitis, facial cellulitis, sepsis, orbital abscess, meningitis, cavernous sinus thrombosis, brain abscess, Lemierre’s syndrome
Odontogenic infections, originating from dental or periodontal tissues, can lead to a wide range of inflammatory complications, from localized abscesses to life-threatening systemic conditions. While periapical abscesses are the most common, severe complications such as sepsis, orbital abscess, meningitis, cavernous sinus thrombosis, brain abscess, and Lemierre’s syndrome pose significant risks due to their potential for rapid progression and high morbidity. These complications arise through various pathways of infection spread, including direct extension into adjacent fascial spaces, hematogenous dissemination, or lymphatic spread, affecting regions such as the maxillary sinus, orbit, mediastinum, and intracranial spaces. This comprehensive review aims to elucidate the etiology, pathogenesis, diagnostic methods, and management strategies for these complications, based on literature from 2005 to 2025, to guide clinical practice and highlight preventive measures.
The aims of this review are:
A systematic literature search was conducted using PubMed, Scopus, and Web of Science, covering peer-reviewed articles published between 2005 and 2025. Search terms included “odontogenic infections,” “periapical abscess,” “subperiosteal abscess,” “submucosal abscess,” “odontogenic sinusitis,” “osteomyelitis of the jaw,” “facial cellulitis,” “odontogenic sepsis,” “orbital abscess,” “meningitis,” “cavernous sinus thrombosis,” “brain abscess,” and “Lemierre’s syndrome.” Inclusion criteria comprised English-language studies focusing on clinical outcomes, case reports, or reviews of odontogenic inflammatory complications. Exclusion criteria included non-peer-reviewed articles, studies on non-odontogenic infections, and those lacking clinical data. After screening titles, abstracts, and full texts, 28 studies were selected for detailed analysis.
The literature search yielded 2,340 articles, of which 28 met inclusion criteria, including 15 original research articles, 10 review articles, and 3 case reports. The studies covered a broad spectrum of complications, including periapical abscesses (periapical, subperiosteal, submucosal), odontogenic sinusitis, osteomyelitis, facial cellulitis, sepsis, orbital abscess, meningitis, cavernous sinus thrombosis, brain abscess, and Lemierre’s syndrome. Clinical studies involved cohorts ranging from 10 to 1,000 patients, primarily in hospital settings. Prevalence varied widely: periapical abscesses accounted for 60–85% of odontogenic infections, while sepsis, orbital abscess, meningitis, cavernous sinus thrombosis, brain abscess, and Lemierre’s syndrome were rare (<1%). Figure 1 illustrates the estimated prevalence of these odontogenic inflammatory complications, highlighting that periapical abscesses account for 60–85% of cases, while rare complications like sepsis or Lemierre’s syndrome occur in less than 1% of cases [1, 19].
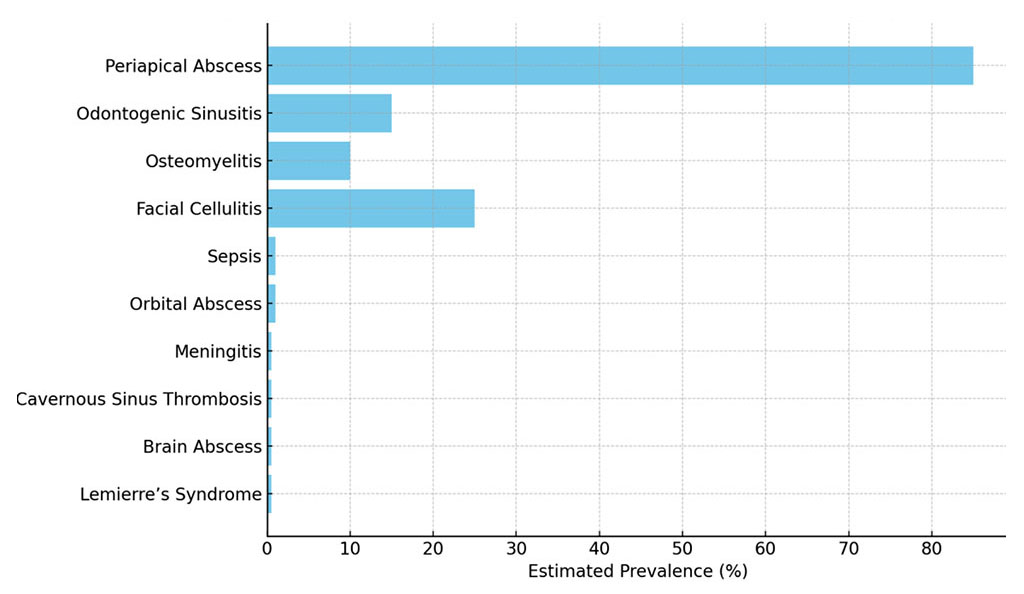
Figure 1. Estimated Prevalence of Odontogenic Inflammatory Complications
Periapical Abscesses
Periapical abscesses arise from bacterial invasion of the dental pulp, primarily due to untreated caries, trauma, or failed endodontic treatment. They are the most common odontogenic infections, accounting for 60–85% of cases [1].
- Periapical Abscess: Localized at the tooth apex, these abscesses present with severe, throbbing pain, swelling, and sometimes a fistulous tract. They result from pulp necrosis, with bacteria such as Streptococcus spp. and anaerobes (e.g., Peptostreptococcus) predominating [2]. Infection typically remains localized but can spread via direct extension into adjacent bone or soft tissues, such as the buccal or vestibular spaces. Chronic cases may lead to osteomyelitis or fistulae to the skin or mucosa.
- Subperiosteal Abscess: This occurs when infection breaches the cortical bone, accumulating beneath the periosteum, causing intense pain, diffuse swelling, and limited mouth opening. Spread occurs through fascial spaces, such as the buccal, submandibular, or masticator spaces, depending on the tooth’s location (e.g., mandibular molars spread to submandibular space, maxillary teeth to buccal space) [3]. Untreated cases may progress to facial cellulitis or deeper spaces [4].
- Submucosal Abscess: Infection extends into submucosal tissues, presenting as a palpable, fluctuant mass with possible drainage through the mucosa. These abscesses are often associated with chronic infections and may involve multiple fascial spaces, such as the canine or palatal spaces [4]. Spread to adjacent structures, such as the maxillary sinus, is possible in maxillary infections.
Odontogenic sinusitis, accounting for 10–15% of maxillary sinusitis cases, results from the spread of infection from maxillary teeth (molars or premolars) to the maxillary sinus via the Schneiderian membrane or direct bone erosion [5]. Symptoms include unilateral facial pain, nasal congestion, purulent discharge, anosmia, and occasionally toothache. The infection may extend to adjacent sinuses (e.g., ethmoid, frontal) via the osteomeatal complex or to the orbit, causing complications like orbital cellulitis or abscess [6]. Common pathogens include anaerobes (e.g., Prevotella) and Streptococcus spp. The proximity of maxillary tooth roots to the sinus floor (often <1 mm) facilitates this spread, particularly in cases of periapical abscesses. Chronic cases may lead to sinus mucosal thickening or polyposis.
Osteomyelitis, a bone marrow infection, occurs in 1–10% of odontogenic infections, predominantly affecting the mandible due to its dense cortical bone and limited vascular supply [7]. It presents with persistent pain, swelling, trismus, and bone sequestration in chronic cases. Infection spreads through the bone marrow, often via the mandibular canal, and can involve adjacent soft tissues or fascial spaces (e.g., submandibular, pterygomandibular, or masseteric spaces). Risk factors include diabetes, immunosuppression, and chronic untreated infections. Chronic osteomyelitis may lead to pathological fractures, fistulae, or spread to the skull base, potentially causing intracranial complications [8]. Acute cases may mimic periapical abscesses, complicating diagnosis.
Facial cellulitis, reported in 15–25% of odontogenic infections, is a diffuse soft tissue infection resulting from untreated dental abscesses or periodontal disease. It spreads through fascial spaces, such as the buccal, submandibular, submental, or masticator spaces, and may progress to Ludwig’s angina, a bilateral infection of the submandibular and sublingual spaces that threatens airway patency [9]. Symptoms include diffuse swelling, erythema, fever, and pain. Polymicrobial infections with anaerobes (e.g., Prevotella, Porphyromonas) and Streptococcus spp. are common [10]. Severe cases may involve the retropharyngeal or danger space, leading to mediastinitis, a life-threatening condition with spread to the mediastinum via the deep cervical fascia. Airway management is critical in advanced cases.
Sepsis, a life-threatening systemic response to infection, occurs in <1% of odontogenic infections, primarily via hematogenous spread from a dental focus. It is more common in immunocompromised patients or those with delayed treatment. Symptoms include fever, tachycardia, hypotension, and multi-organ dysfunction (e.g., respiratory or renal failure). The infection may disseminate to distant sites, including the lungs (septic emboli), liver, or brain, increasing mortality risk [11,12]. Mediastinitis, a rare but severe complication, occurs via the retropharyngeal space or deep cervical fascia, presenting with chest pain, dyspnea, and systemic toxicity. Early recognition and aggressive treatment are essential.
Orbital abscess (<1%) results from the spread of infection from the maxillary sinus or canine space to the orbit, often via the ethmoidal veins or direct extension through the orbital floor. Symptoms include periorbital swelling, pain, proptosis, restricted eye movement, and vision impairment. Untreated cases can lead to blindness, cavernous sinus thrombosis, or intracranial spread [13,14]. The orbit’s anatomical proximity to the maxillary sinus and lack of lymphatic drainage facilitate rapid infection spread. Risk factors include chronic sinusitis and delayed treatment of dental infections.
Odontogenic meningitis (<0.5%) is a rare but severe complication, typically resulting from hematogenous spread or direct extension through the skull base. Symptoms include fever, neck stiffness, headache, photophobia, and altered consciousness. The infection may reach the meninges via the cavernous sinus, pterygoid plexus, or direct erosion from the maxilla or mandible [15,16]. Maxillary infections are particularly implicated due to their proximity to intracranial structures. The high mortality rate necessitates urgent diagnosis and treatment.
Cavernous sinus thrombosis (<0.5%) occurs when infection spreads via the valveless venous sinuses, particularly from maxillary teeth or the canine space. Symptoms include severe headache, fever, cranial nerve palsies (III, IV, V, VI), bilateral proptosis, and chemosis. The pterygoid plexus and facial veins are common pathways of spread, making this a life-threatening condition [17]. Rapid progression can lead to intracranial complications, including meningitis or brain abscess. Early intervention is critical to prevent permanent neurological damage.
Brain abscess (<0.5%) develops through hematogenous spread or direct extension from an odontogenic focus, often involving the frontal or temporal lobes due to their proximity to the maxilla. Symptoms include headache, fever, seizures, and focal neurological deficits (e.g., hemiparesis, aphasia). The infection may spread via the bloodstream or through contiguous spaces, such as the maxillary sinus or middle cranial fossa [16]. Prognosis is poor without prompt surgical drainage, and residual neurological deficits are common.
Lemierre’s syndrome (<0.5%) is a rare complication characterized by septic thrombophlebitis of the internal jugular vein, typically caused by Fusobacterium necrophorum. It presents with fever, neck pain, swelling, and septic pulmonary emboli, manifesting as cough, hemoptysis, or chest pain. The infection spreads hematogenously from a dental or oropharyngeal focus, often via the tonsillar veins [18]. Delayed treatment can lead to systemic dissemination, multi-organ failure, and high mortality. The tonsillar and peritonsillar spaces are key pathways for spread.
The development of odontogenic inflammatory complications is a complex process driven by a combination of etiological factors and intricate pathogenic mechanisms that facilitate the initiation, progression, and spread of infection. These factors include dental pathologies, microbial dynamics, host-related predispositions, and iatrogenic influences, all of which contribute to the severity and potential complications of odontogenic infections. Understanding these elements is critical for effective diagnosis and management, as the infection can spread through multiple pathways, leading to localized or systemic consequences, some of which may be life-threatening.
Dental pathologies serve as the primary initiators of odontogenic infections. Conditions such as untreated dental caries, pulpitis, dental trauma, or periodontal disease create an environment conducive to bacterial invasion. Caries, if left unaddressed, can progress to involve the dental pulp, leading to pulpitis, while trauma or periodontal disease may compromise the integrity of the periodontium. These conditions allow bacteria to penetrate the pulp or surrounding periodontal tissues, establishing a foothold for infection and potentially leading to complications such as abscesses or osteomyelitis [1].
Microbial factors play a central role in the pathogenesis of odontogenic infections, which are typically polymicrobial in nature. The infections are predominantly caused by a mix of aerobic and anaerobic bacteria, with Streptococcus species, Staphylococcus species, and anaerobic organisms such as Prevotella, Porphyromonas, and Fusobacterium being commonly implicated. The synergistic interaction between aerobic and anaerobic bacteria enhances their collective virulence, promoting more aggressive tissue destruction and complicating treatment efforts. This microbial synergy enables the infection to overcome host defenses, leading to rapid progression and spread if not adequately addressed [2].
Host-related factors significantly influence the susceptibility to and severity of odontogenic infections. Systemic conditions such as diabetes mellitus, immunosuppression (e.g., due to HIV/AIDS or chemotherapy), malnutrition, or poor oral hygiene impair the body’s ability to mount an effective immune response. These conditions create a favorable environment for bacterial proliferation and increase the likelihood of severe complications, as the host’s compromised defenses struggle to contain the infection [7].
Iatrogenic factors can further exacerbate odontogenic infections, contributing to their persistence or worsening. Inadequate endodontic treatment, such as incomplete root canal therapy, can leave residual infection, allowing bacteria to persist and spread. Delayed surgical intervention, such as failure to drain an abscess promptly, may permit the infection to extend into deeper tissues or fascial spaces. Additionally, inappropriate or indiscriminate use of antibiotics, such as prescribing ineffective agents or failing to account for bacterial resistance, can lead to treatment failure and worsening of the infection [3].
The pathways of infection spread are critical to understanding the potential complications of odontogenic infections. Table 1 summarizes the pathways of spread of odontogenic infections, illustrating primary routes of local and systemic dissemination [2, 7, 9, 16, 18, 20].
Table 1. Pathways of Spread of Odontogenic Infections
| Pathway of Spread | Description | Examples |
| Direct Extension | Local spread through fascial spaces or bone erosion, often leading to adjacent tissue involvement [7, 8]. | Buccal, submandibular, pterygomandibular, masseteric, retropharyngeal, and danger spaces; maxillary sinus, orbital floor, skull base. |
| Hematogenous Spread | Dissemination via bloodstream to distant organs, resulting in severe systemic complications [9, 19]. | Brain (abscess, meningitis), lungs (empyema), mediastinum (mediastinitis), liver (abscess). |
| Lymphatic Spread | Spread through regional lymph nodes, facilitating systemic dissemination and rare complications [16, 25]. | Sepsis, Lemierre’s syndrome (internal jugular vein thrombosis). |
Caption: Pathways of spread of odontogenic infections, illustrating primary routes of local and systemic dissemination based on literature data [7, 8, 9, 16, 19, 25].
Direct extension is a common route, where the infection spreads through fascial spaces, such as the buccal, submandibular, pterygomandibular, masseteric, retropharyngeal, or danger spaces, or via bone erosion affecting structures like the maxillary sinus, orbital floor, or skull base. This direct spread can lead to localized complications such as cellulitis or abscess formation in adjacent tissues. Hematogenous spread occurs when bacteria enter the bloodstream, disseminating to distant sites such as the brain, lungs, mediastinum, or liver, potentially causing severe complications like brain abscesses or mediastinitis. Lymphatic spread involves the regional lymph nodes and can contribute to systemic dissemination, particularly in severe cases such as sepsis or Lemierre’s syndrome, where the infection spreads rapidly and poses significant risks to the patient’s life [2, 7]. Figure 3 illustrates these pathways, highlighting the complexity of odontogenic infections [2, 7, 9, 16, 18, 20].
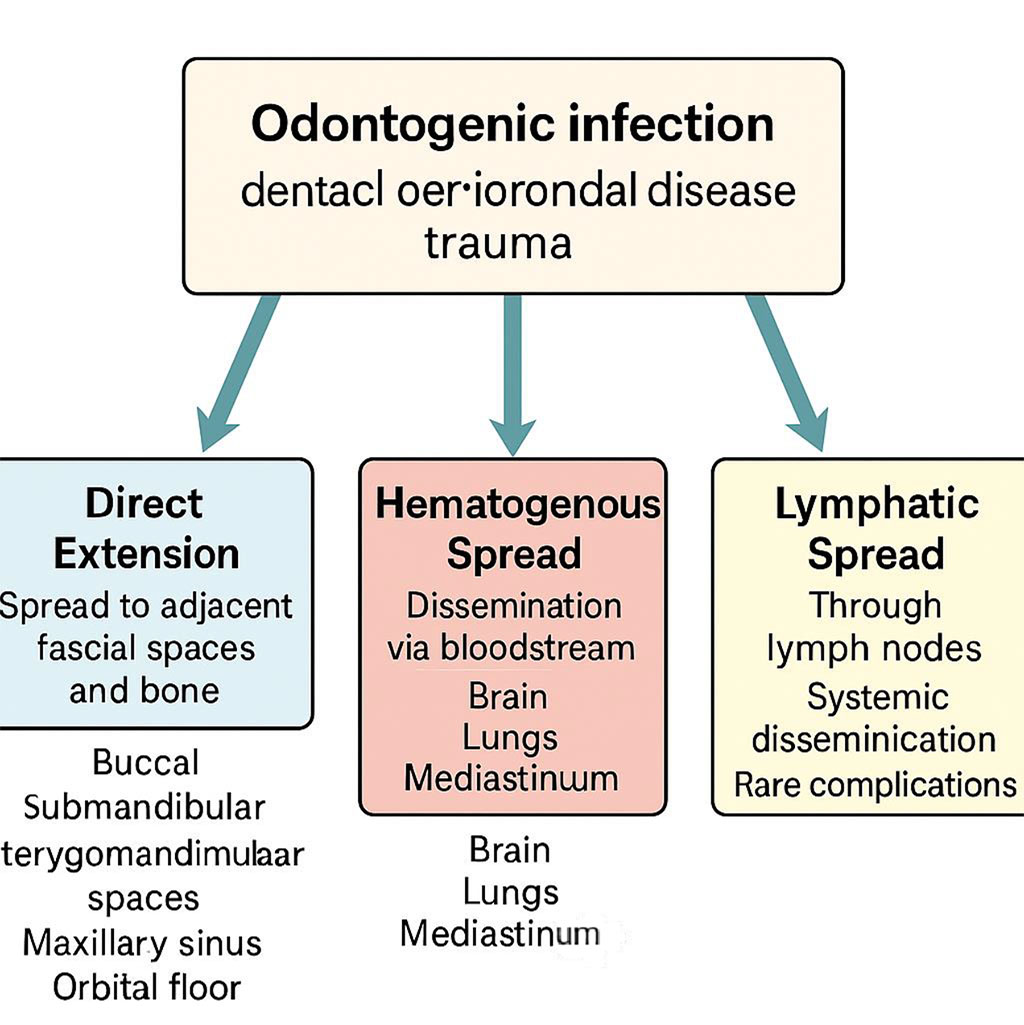
Figure 3. Pathways of Spread of Odontogenic Infections
The diagnostic approach to odontogenic infections and their complications relies on a comprehensive evaluation that integrates clinical examination, advanced imaging techniques, and microbiological testing to ensure accurate identification and management of the condition. Clinical examination plays a pivotal role in the initial diagnosis, focusing on the identification of symptoms that may indicate the presence and severity of infection. Common clinical manifestations include localized pain, which may be sharp or throbbing, and swelling, which can vary from mild edema to significant tissue distension. Fever is frequently observed, reflecting the systemic inflammatory response to infection. Trismus, or restricted jaw movement, often accompanies infections that affect the masticatory muscles or adjacent structures. In cases involving orbital complications, proptosis protrusion of the eyeball may be evident, while neurological symptoms such as neck stiffness or cranial nerve palsies can suggest more severe intracranial involvement. Specific clinical signs are particularly critical for identifying life-threatening conditions. For instance, bilateral proptosis is a hallmark of cavernous sinus thrombosis, a rare but serious complication, while seizures may point to the development of a brain abscess, necessitating urgent diagnostic and therapeutic intervention [4]. Table 2 summarizes the clinical features and diagnostic methods for odontogenic inflammatory complications [4, 5, 6, 7, 9, 11, 13, 14, 15, 16, 18, 21].
Table 2. Clinical Features of Odontogenic Inflammatory Complications
| Complication | Clinical Features | Diagnostic Methods | References |
| Periapical Abscess | Localized pain, swelling, erythema, pus discharge, tooth sensitivity | Clinical examination, panoramic radiograph, CBCT | [4, 26] |
| Odontogenic Sinusitis | Nasal congestion, facial pain, purulent rhinorrhea, dental pain | Nasal endoscopy, CT, CBCT | [5, 20] |
| Osteomyelitis | Chronic jaw pain, swelling, bone destruction, sequestra | CBCT, MRI, bone biopsy | [6, 21] |
| Facial Cellulitis | Diffuse swelling, erythema, fever, trismus | Clinical examination, ultrasound, CT | [8] |
| Sepsis | Fever, tachycardia, hypotension, systemic inflammatory response | Blood cultures, CRP, procalcitonin | [9, 19] |
| Orbital Abscess | Proptosis, ophthalmoplegia, vision impairment, periorbital swelling | CT, MRI, ophthalmologic evaluation | [11, 22] |
| Meningitis | Headache, neck stiffness, fever, altered mental status | Lumbar puncture, MRI, CT | [13] |
| Cavernous Sinus Thrombosis | Severe headache, cranial nerve deficits, proptosis | MRI, CT venography | [15, 24] |
| Brain Abscess | Headache, seizures, focal neurological deficits | MRI, CT, neurosurgical consultation | [14] |
| Lemierre’s Syndrome | Sore throat, neck pain, internal jugular vein thrombosis, septic emboli | Ultrasound, CT, blood cultures | [16, 25] |
Caption: Summary of clinical features and diagnostic methods for odontogenic inflammatory complications, based on literature data [4, 5, 6, 8, 9, 11, 13, 14, 15, 16, 19, 20, 21, 22, 24, 25, 26].
Imaging studies are indispensable for confirming the clinical diagnosis and delineating the extent of infection, particularly when it involves bone, soft tissues, or intracranial structures. Panoramic radiographs provide a broad overview of the maxillofacial region and are commonly used as an initial imaging modality to detect abnormalities such as periapical abscesses or osteomyelitis. Cone-beam computed tomography (CBCT) offers a more detailed, three-dimensional visualization of bone and soft tissue structures, making it a standard tool for assessing conditions like periapical abscesses, osteomyelitis, and odontogenic sinusitis. CBCT’s high resolution allows clinicians to evaluate the precise location and extent of bone destruction or sinus involvement, which is critical for planning treatment. For complications involving soft tissues or intracranial structures, magnetic resonance imaging (MRI) is the preferred modality due to its superior ability to resolve soft tissue details. MRI is particularly valuable in diagnosing conditions such as orbital abscess, meningitis, brain abscess, and cavernous sinus thrombosis, where precise visualization of soft tissue inflammation, fluid collections, or vascular involvement is essential for accurate diagnosis and management [6, 8]. Recent studies have highlighted the role of advanced imaging techniques, such as diffusion-weighted MRI, in improving the detection of abscesses and differentiating them from other lesions, enhancing diagnostic precision in complex cases [21]. Figure 2 provides a diagnostic algorithm for odontogenic infections, outlining a stepwise approach to identifying localized and systemic complications [4, 5, 6, 7, 9, 11, 13, 14, 15, 16, 18, 21].
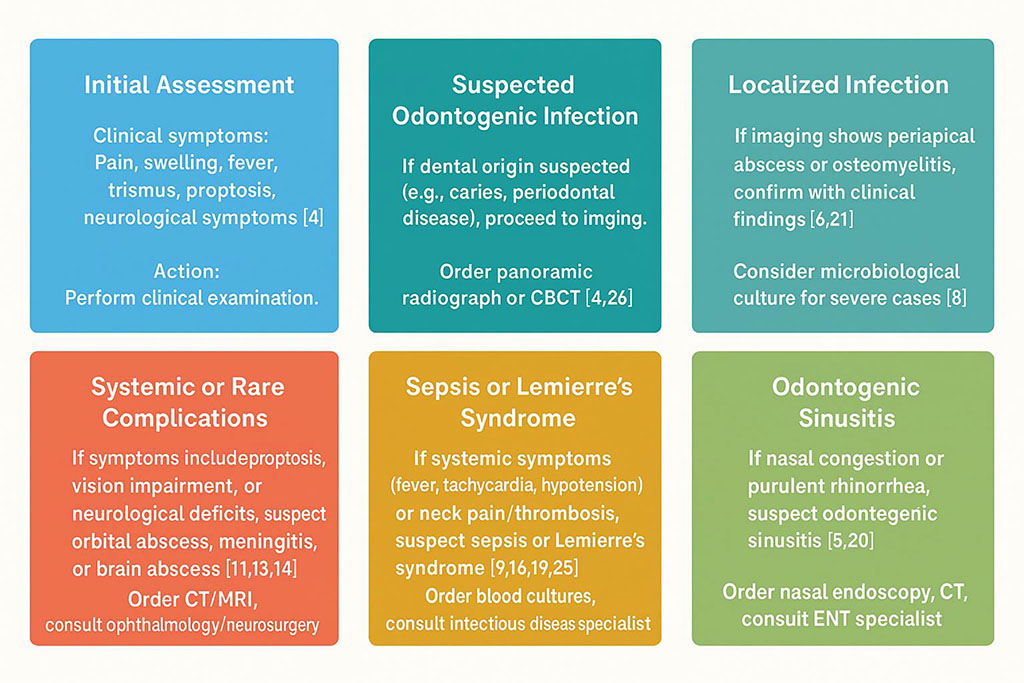
Figure 2. Diagnostic Algorithm for Odontogenic Infections
Microbiological testing is a cornerstone of the diagnostic process, especially in severe or complicated infections where precise identification of the causative pathogens is necessary to guide effective treatment. Cultures and antibiograms, which assess the antibiotic susceptibility of isolated pathogens, are critical in cases such as sepsis, brain abscess, or Lemierre’s syndrome, where delayed or inappropriate treatment can lead to catastrophic outcomes. These tests are particularly important in odontogenic infections, which are often polymicrobial, involving a mix of aerobic and anaerobic bacteria. By identifying the specific pathogens and their antibiotic resistance profiles, microbiological testing enables clinicians to tailor antibiotic therapy, ensuring optimal efficacy and minimizing the risk of treatment failure or the development of resistant strains. This approach is especially vital in severe infections, where the complexity of the microbial flora and the potential for rapid clinical deterioration necessitate a targeted and evidence-based treatment strategy [10]. Emerging molecular diagnostic tools, such as polymerase chain reaction (PCR) assays, have shown promise in rapidly identifying pathogens in odontogenic infections, particularly anaerobes, improving diagnostic turnaround time [22].
The management of odontogenic infections requires a comprehensive therapeutic approach, which includes both pharmacological interventions and surgical procedures, often coordinated by a multidisciplinary medical team. Early and adequate treatment is crucial to prevent the progression of local infections into life-threatening systemic complications. Table 3 summarizes the treatment strategies for odontogenic inflammatory complications, detailing antimicrobial and surgical approaches [4, 5, 6, 7, 9, 10, 11, 13, 14, 15, 16, 18, 23].
Table 3. Treatment Strategies for Odontogenic Inflammatory Complications
| Complication | Antimicrobial Therapy | Surgical Interventions | References |
| Periapical Abscess | Amoxicillin-clavulanate, clindamycin | Drainage, tooth extraction | [4, 23] |
| Odontogenic Sinusitis | Amoxicillin, doxycycline | Endoscopic sinus surgery, dental treatment | [5, 20] |
| Osteomyelitis | IV antibiotics (ceftriaxone, vancomycin), prolonged therapy | Debridement, sequestrectomy | [6, 21] |
| Facial Cellulitis | Penicillin, metronidazole | Incision and drainage | [8] |
| Sepsis | Broad-spectrum IV antibiotics (ceftriaxone, vancomycin, metronidazole) | Source control, ICU care | [9, 19] |
| Orbital Abscess | IV antibiotics (ceftriaxone, vancomycin) | Endoscopic drainage, orbitotomy | [11, 22] |
| Meningitis | Ceftriaxone, vancomycin, steroids | Neurosurgical consultation | [13] |
| Cavernous Sinus Thrombosis | IV antibiotics, anticoagulation | Surgical drainage if needed | [15, 24] |
| Brain Abscess | IV antibiotics (ceftriaxone, metronidazole) | Neurosurgical drainage | [14] |
| Lemierre’s Syndrome | IV antibiotics (penicillin, metronidazole), anticoagulation | Thrombectomy in severe cases | [16, 25] |
Caption: Overview of antimicrobial and surgical treatment strategies for odontogenic inflammatory complications, based on literature data [4, 5, 6, 8, 9, 11, 13, 14, 15, 16, 19, 20, 21, 22, 23, 24, 25].
Antimicrobial therapy forms the cornerstone of initial management. In cases of mild infections, such as uncomplicated periapical abscesses, empirical antibiotic treatment with amoxicillin-clavulanate or metronidazole is commonly prescribed for a duration of 7 to 14 days. This regimen targets the characteristic polymicrobial flora involved in odontogenic infections, consisting of both aerobic and anaerobic bacteria [5]. Prompt antibiotic administration not only accelerates symptom resolution but also helps to limit the spread of infection to adjacent anatomical structures.
In contrast, severe odontogenic infections including sepsis, orbital abscess, meningitis, cavernous sinus thrombosis, brain abscess, and Lemierre’s syndrome require immediate hospitalization and intravenous antibiotic therapy. In these situations, broad-spectrum antibiotics such as ceftriaxone, vancomycin, and metronidazole are administered, often in combination, and are subsequently adjusted based on culture and sensitivity results. The duration of therapy in such advanced cases typically extends to 4–6 weeks, reflecting the complexity and severity of these conditions [11, 18]. Recent studies advocate for the use of adjunctive therapies, such as hyperbaric oxygen therapy, in severe cases like osteomyelitis or necrotizing infections, to enhance tissue oxygenation and antimicrobial efficacy [23].
Surgical interventions are frequently indispensable for effective infection control, particularly when abscesses have formed. Drainage of purulent collections, whether periapical, subperiosteal, or submucosal, is performed through incision, aspiration, or trephination, allowing decompression and removal of infectious material [3]. In many cases, extraction of the causative tooth is necessary, especially when endodontic treatment proves unsuccessful or is not feasible due to extensive destruction of dental structures [1]. Minimally invasive techniques, such as laser-assisted drainage, have shown promise in reducing tissue trauma and improving recovery times in localized abscesses [24].
More invasive procedures may be warranted in complicated scenarios. For instance, osteomyelitis often requires surgical debridement to remove necrotic bone tissue and prevent further spread of the infection. Orbital abscesses may necessitate endoscopic drainage to relieve orbital pressure and preserve visual function, while brain abscesses often require neurosurgical drainage to alleviate intracranial pressure and prevent neurological deterioration [8, 13, 16].
In cases of early periapical infections, root canal therapy remains an effective conservative treatment option. By eliminating the source of infection within the root canal system, this approach prevents disease progression and preserves the affected tooth whenever possible [1].
The management of severe odontogenic infections frequently demands an interdisciplinary approach. Collaboration with otolaryngologists is essential for the treatment of sinusitis or orbital abscesses, while neurosurgeons play a critical role in addressing complications such as meningitis or brain abscesses. Infectious disease specialists are integral to the management of complex systemic infections, including sepsis and Lemierre’s syndrome [14, 15, 17]. Moreover, in cases involving airway compromise, such as Ludwig’s angina or mediastinitis, securing the airway takes absolute priority to ensure patient survival. Recent advances in multidisciplinary protocols have improved outcomes in complex cases by standardizing collaboration across specialties [25].
Prevention of odontogenic infections requires a comprehensive and multifactorial approach that encompasses both individual patient care and public health strategies. Regular dental check-ups play a crucial role in the early identification and management of initial pathological changes within the oral cavity, thereby significantly reducing the incidence of odontogenic infections [17]. During these routine visits, dental professionals can monitor the condition of teeth and periodontal tissues, detect early signs of caries, pulpitis, or gingivitis, and implement timely interventions before these conditions progress to more severe complications [3]. Table 4 summarizes key preventive measures for odontogenic infections, highlighting strategies to reduce incidence and complications [3, 17, 26].
Table 4. Preventive Measures for Odontogenic Infections
| Measure | Description | Target Population | References |
| Regular Dental Check-ups | Routine examinations to detect caries and periodontal disease early | General population | [7, 17] |
| Oral Hygiene Education | Promoting brushing, flossing, and fluoride use | Children, adults | [17, 28] |
| Systemic Disease Management | Control of diabetes, immunosuppression to reduce infection risk | High-risk patients | [7, 27] |
| Public Health Campaigns | Community programs on caries prevention and oral health | General population | [28] |
| Early Intervention | Prompt treatment of dental infections to prevent complications | Patients with early symptoms | [17, 23] |
Caption: Key preventive measures for reducing the incidence of odontogenic infections and their complications, based on literature data [7, 17, 23, 27, 28].
In addition to clinical management, patient education on proper oral hygiene practices constitutes a cornerstone of prevention. Instruction on effective brushing techniques, interdental cleaning, and the use of antibacterial mouth rinses can substantially limit the accumulation of dental plaque, a primary etiological factor for caries and periodontal diseases. Consequently, by controlling these primary infections, the likelihood of secondary, more serious odontogenic complications is markedly diminished [3, 17]. Community-based programs promoting fluoride use and dietary counseling have been shown to further reduce the incidence of dental caries, particularly in high-risk populations [26].
Moreover, the management of systemic health conditions such as diabetes mellitus, immunosuppressive disorders, or any state of compromised immunity is essential for reducing susceptibility to odontogenic infections. Individuals with poorly controlled systemic diseases often exhibit impaired immune responses, which facilitate bacterial colonization and dissemination from odontogenic foci. Optimizing the control of these systemic conditions can therefore enhance the host’s defense mechanisms, lowering the risk of both localized and systemic complications arising from odontogenic infections [7]. Table 5 summarizes key risk factors contributing to odontogenic inflammatory complications, emphasizing the importance of targeted prevention [2, 3, 4, 7, 9, 15, 16, 17, 27].
Table 5. Risk Factors for Odontogenic Inflammatory Complications
| Risk Factor | Description | Associated Complications | References |
| Poor Oral Hygiene | Inadequate brushing, flossing, or dental care | Periapical abscess, odontogenic sinusitis, osteomyelitis | [7, 17] |
| Untreated Dental Caries | Progression of caries to pulpitis or periapical infection | Periapical abscess, facial cellulitis | [4, 8] |
| Periodontal Disease | Chronic gingival inflammation leading to bone loss | Osteomyelitis, sepsis | [6, 19] |
| Immunosuppression | Compromised immune response (e.g., diabetes, HIV) | Sepsis, orbital abscess, brain abscess | [9, 27] |
| Delayed Treatment | Late intervention for dental infections | Cavernous sinus thrombosis, Lemierre’s syndrome | [15, 25] |
| Smoking | Impaired healing and increased infection risk | Osteomyelitis, facial cellulitis | [17, 19] |
Caption: Summary of key risk factors contributing to the development of odontogenic inflammatory complications, based on literature data [4, 6, 7, 8, 9, 15, 17, 19, 25, 27].
On a broader scale, public health campaigns aimed at promoting oral health awareness have the potential to significantly decrease the burden of odontogenic complications. Educational programs targeting various populations—from school-aged children to the elderly—can foster lifelong habits that support oral health maintenance. These initiatives may include community-based fluoride programs, nutritional counseling to reduce sugar intake, and easy access to preventive dental services, all of which contribute to minimizing the incidence of oral infections and their sequelae [17]. Recent global health initiatives have emphasized the integration of oral health into primary care systems to enhance access and reduce disparities [28].
The literature highlights the diverse spectrum of odontogenic inflammatory complications, ranging from common periapical abscesses to rare, life-threatening conditions like sepsis, meningitis, cavernous sinus thrombosis, brain abscess, and Lemierre’s syndrome [1, 18]. Periapical abscesses, including subperiosteal and submucosal types, are the most frequent but can serve as gateways to severe complications through direct extension (e.g., into buccal, submandibular, or retropharyngeal spaces), hematogenous spread (e.g., to brain, lungs, mediastinum), or lymphatic dissemination [2, 7, 9]. The maxillary sinus, orbit, mediastinum, and intracranial spaces are particularly vulnerable due to anatomical proximity and vascular connections [5, 11, 15]. Advances in imaging, such as CBCT for bone assessment and MRI for soft tissue evaluation, have improved diagnostic accuracy, enabling early detection [6, 21]. Targeted antimicrobial therapy, combined with surgical interventions like drainage or extraction, remains the cornerstone of treatment [4, 10, 23]. Preventive strategies, including patient education, early dental care, and management of systemic risk factors, are critical to reducing incidence [17, 27]. Interdisciplinary collaboration is vital for managing severe cases, particularly those involving the orbit, brain, or systemic dissemination [13, 15, 25].
Recent studies have further elucidated the role of emerging diagnostic tools, such as biomarkers for early detection of odontogenic infections, which could enhance diagnostic precision and guide timely intervention [19]. Additionally, the increasing prevalence of antibiotic-resistant strains in odontogenic infections underscores the need for antibiotic stewardship programs to optimize treatment outcomes [20]. Advances in minimally invasive surgical techniques, such as laser-assisted procedures, offer potential for reducing morbidity and improving recovery times [24]. Furthermore, epidemiological data suggest that socioeconomic factors, such as limited access to dental care, significantly contribute to the incidence of severe odontogenic complications, particularly in underserved populations [28]. Future research should focus on optimizing antibiotic stewardship to combat resistance, developing minimally invasive surgical techniques, and exploring novel diagnostic tools, such as biomarkers for early infection detection, to improve outcomes and reduce morbidity.
The literature on odontogenic inflammatory complications highlights advancements and ongoing challenges in diagnosis, treatment, and prevention. A key controversy involves imaging modalities for rare complications. Cone-beam computed tomography (CBCT) effectively detects bone involvement in periapical abscesses and osteomyelitis [6], while magnetic resonance imaging (MRI) is preferred for soft tissue complications like orbital abscess or meningitis [11, 13]. Limited MRI access in some settings may delay severe case diagnosis [6]. Recent studies advocate diffusion-weighted MRI to differentiate abscesses from other lesions, improving diagnostic accuracy [21].
Therapeutic challenges are significant. Treatment strategies emphasize antimicrobial therapy and surgical interventions [4, 5, 6, 7, 9, 10, 11, 13, 14, 15, 16, 18, 23]. Antibiotic resistance, particularly in severe infections like sepsis or Lemierre’s syndrome, poses a growing concern [10]. Studies promote antibiotic stewardship to optimize therapy, but the lack of standardized protocols for complex cases complicates management [10]. Novel therapies, such as advanced antimicrobial agents or minimally invasive surgical techniques, are underrepresented in the literature, limiting clinical integration [10, 24]. Hyperbaric oxygen therapy for osteomyelitis shows promise but requires further validation [23]. This gap is notable in pediatric populations, where treatment efficacy data are scarce [9].
Prevention remains critical, encompassing strategies like regular dental check-ups and public health campaigns [3, 17, 26]. Risk factors, such as poor oral hygiene and immunosuppression, increase complication likelihood, emphasizing targeted interventions [2, 3, 4, 7, 9, 15, 16, 17, 27]. Pathways of infection spread, from local extension to systemic dissemination, highlight the complexity of odontogenic infections [2, 7, 9, 16, 18, 20]. Preventive measure effectiveness varies across populations, particularly in underserved regions with limited dental care access [17, 28]. High prevalence of periapical abscesses (60–85%) compared to rare complications (<1%) underscores the need for targeted prevention to reduce common infection burden [1, 19].
Significant literature gaps include limited data on pediatric and immunocompromised populations, as noted in the study limitations section. Study design heterogeneity and lack of meta-analyses hinder quantitative synthesis of prevalence and outcomes [9, 16]. Emerging research highlights molecular diagnostics, such as PCR-based assays, to improve pathogen identification and guide treatment in complex cases [22]. Future research should prioritize standardized reporting, diverse population inclusion, and novel therapy evaluation. Interdisciplinary collaboration, especially for severe complications like brain abscess or cavernous sinus thrombosis, is essential to improve outcomes [13, 15, 25].
While this literature review followed a structured methodology, several limitations must be acknowledged, as they may affect the interpretation and generalizability of the findings:
Future research should consider incorporating non-English literature, conducting meta-analyses where feasible, exploring standardized protocols for reporting clinical outcomes, and including diverse populations and novel therapeutic approaches to enhance comparability and generalizability.
Odontogenic infections remain a frequent but potentially severe medical concern, capable of progressing to life-threatening complications if left untreated. While advancements in diagnostic imaging, antimicrobial therapy, and surgical management have improved clinical outcomes, challenges persist—particularly in cases involving systemic dissemination, antibiotic resistance, and limited access to care. Preventive strategies, early intervention, and interdisciplinary collaboration are critical in mitigating risks and reducing morbidity. Future research should focus on standardizing treatment protocols, incorporating novel diagnostic and therapeutic approaches, and addressing gaps in data on vulnerable populations.
Conceptualization: Hubert Knapik, Katarzyna Janik, Maja Borowska
Methodology: Hubert Knapik, Julia Podgórska, Adam Białobłocki
Investigation: Hubert Knapik, Maja Borowska, Julia Podgórska, Kornelia Kustra, Jakub Rafalski
Data curation: Katarzyna Janik, Adam Białobłocki, Anna Klukowska, Kamil Barszcz
Writing – original draft: Hubert Knapik, Julia Podgórska, Jakub Rafalski, Anna Klukowska, Jakub Klimkiewicz
Writing – review and editing: Hubert Knapik, Katarzyna Janik, Maja Borowska, Kornelia Kustra, Kamil Barszcz
Validation: Hubert Knapik, Adam Białobłocki, Jakub Rafalski, Jakub Klimkiewicz
Visualization: Maja Borowska, Julia Podgórska, Anna Klukowska
Supervision: Hubert Knapik, Katarzyna Janik
Project administration: Hubert Knapik, Kamil Barszcz
The authors acknowledge the use of artificial intelligence tools, Grok (created by xAI) and ChatGPT (created by OpenAI), for assistance in drafting initial versions of certain sections and polishing the language of the manuscript. These tools were used to enhance clarity and organization of the text. All content was thoroughly reviewed, edited, and validated by the authors to ensure scientific accuracy, integrity, and alignment with the study’s objectives.
The authors declare no conflicts of interest.