- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Aim: The aim of the study is to identify the causes of fractures of partially mobile skeletal prostheses, to determine the technological register or the design aspects of the prosthetic solution according to the particularity of the clinical case. Methods: A total of 56 fractured, partially removable skeletal prostheses were analyzed by AFM (Atomic Force Microscopy) between 2019 and 2023 to determine the structural changes in the fractured area compared to the other areas of the prosthesis. The surface images were obtained with a Solver PRO-M scanning probe microscope (NT-MDT), in AFM configuration. Results: According to the results in the literature by comparing the fatigue limit on different criteria it was revealed that the surface roughness was a significant factor in the material performance and with a high influence on the fatigue strength of the specimen. The surface roughness in the fracture zone, is much higher than the surfaces in the areas that resisted external stresses. Conclusions: Usually, the failure of a metal material in operation is due to a combination of factors, which are found in the technological register or may be generated by the particularities of the clinical case that have not been taken into account.
Keywords: fractures, skeletal removable partial dentures, Atomic Force Microscopy, surface roughness
Extensive partial edentulism is a complex clinical entity with profound local, loco-regional and general implications through the related conditions that can be triggered.
Its individuality lies in the particularity of the clinical case in conjunction with socio-economic and clinical-technical criteria [4,6].
Contemporary dental medicine offers patients diagnosed with partially edentulous teeth a variety of therapeutic solutions, in which implant-prosthetic rehabilitations occupy an important place, with significant costs for the patient, while it is known that there is a variety of therapeutic solutions represented by partially removable skeletal prostheses [1,2]. These types of prostheses have a high degree of precision, ensuring optimal functionality and aesthetics [7,19].
Partially removable skeletal prostheses are characterized by a very good adaptation in most clinical cases of partially edentulous teeth, an adaptation based on the correlation between the particularity of the clinical case, the variety of the design, the choice of the type of alloy, without circumventing the socio-economic criteria, so as to result in resistant prosthetic substitutes that ensure static and dynamic balance in relation to the physiological capacity to take up the forces during mastication [8,16]. The most widely used metal alloys for the structure of partially removable skeletal prostheses are Cobalt Chromium alloys, which have optimal properties for ensuring the rigidity of the final prosthetic construction, high bending strength and a high degree of biocompatibility [3,4]. The mechanical properties of the Cobalt-Chromium alloy in conjunction with the casting technique form the basis for the planning and fabrication of the components of partially removable skeletal prostheses, so that they can have an optimal biomechanical behavior, avoiding fractures or deformation [10,14].
A number of authors have described the presence of structural defects such as voids, porosities and inclusions that may be the cause of various subsequent fractures of the removable prosthesis.
Fatigue is another form of deficiency that can occur in the biomechanical behavior of partially removable skeletal prostheses leading to fracture through loss of mechanical properties of biomaterials under the action of repetitive forces [12,13,17].
The structure of partially removable prostheses according to the particularities of the clinical case may suffer fractures caused by alloy fatigue especially during the setting and unsetting of the prosthesis. It is particularly important to identify the causes of prosthesis fracture, some of which are strictly related to the design and technological elements, while others are related to the clinical context, to the supraliminal forces that have not been correctly assessed, to the type of static and especially dynamic occlusion [20,21].
The aim of the study is to identify the causes of fractures of partially mobile skeletal prostheses, to determine the technological register or the design aspects of the prosthetic solution according to the particularity of the clinical case.
A total of 56 fractured, partially removable skeletal prostheses were analyzed by AFM (Atomic Force Microscopy) between 2019 and 2023 to determine the structural changes in the fractured area compared to the other areas of the prosthesis. The surface images were obtained with a Solver PRO-M scanning probe microscope (NT-MDT, Russia), in AFM configuration. Rectangular silicon cantilevers NSG10 (NT-MDT, Russia) with tips of high aspect ratio (sharpened pyramidal tip, angle of nearly 20o, tip curvature radius of 10 nm and height of 14–16 µm) were used in order to minimize convolution effects. All images were acquired in air, at room temperature(23oC), in tapping mode, with the velocity of 6 mm/s. For image acquisition, Nova v.19891 for Solver software was used.
We notice fracture risk main connector-sells junction 36%, fracture risk clasps-active arm 30%, Fracture risk clasps-opposite arm 15% and 10% for secondary connector and 9% fracture risk for connector (Fig.1.)
Regarding the causes of fracture of different components of skeletal removable prostheses, we remark 45% for technological deficiencies, 28% for clinical deficiencies, 20% for deficiencies in planning and design, and 7 % caused by bruxism (Fig. 2).
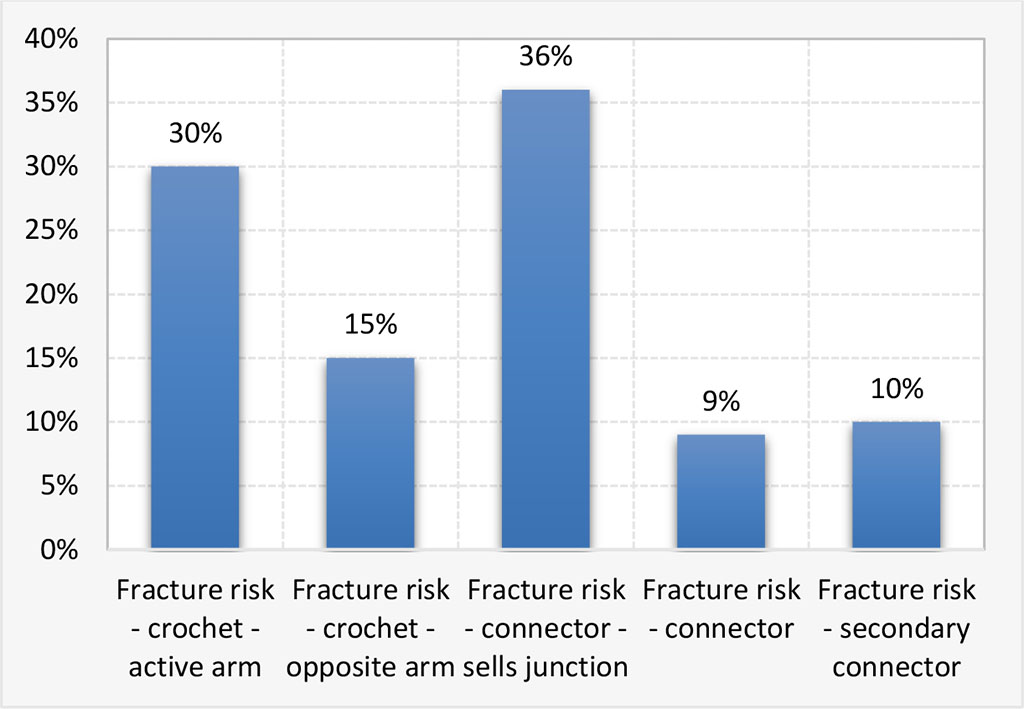
Figure 1. Risk fracture for skeletal removable prostheses components
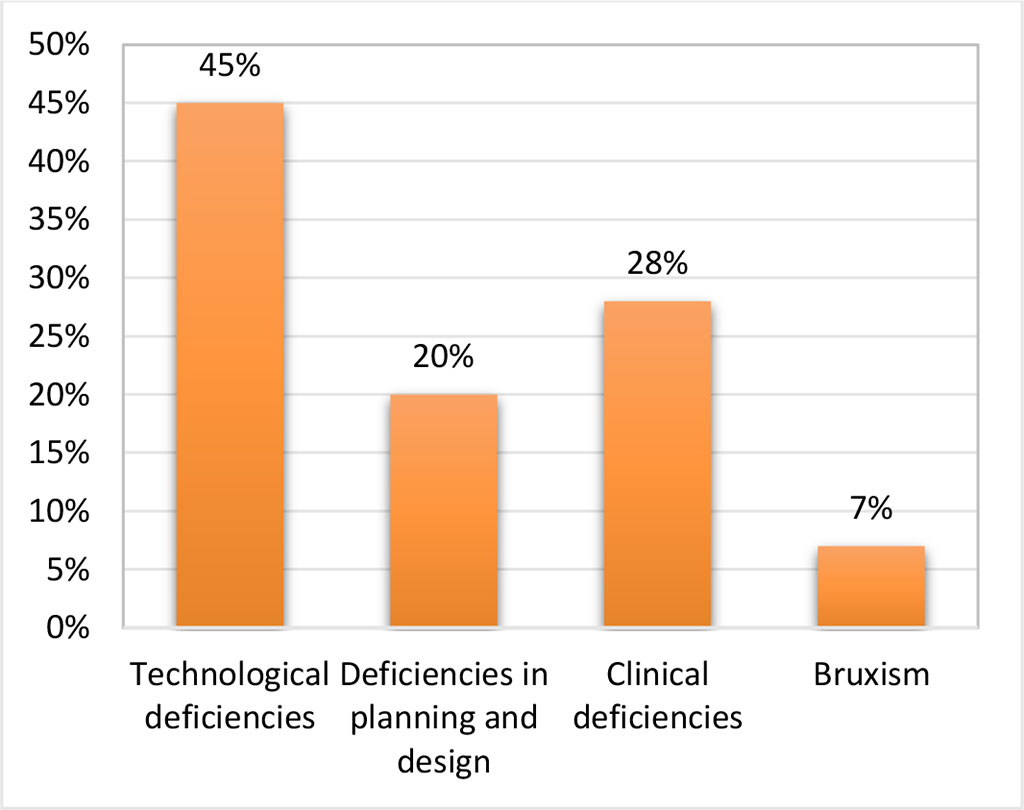
Figure 2. The causes involved in fracture of skeletal removable prostheses
AFM scans were performed on various areas of a dental element that broke in operation. The main roughness for different scanned surfaces is given in Table 1.
Table 1. Surface roughness of dental metal elements
| 20x20 | 40x40 | 60x60 | |
| 1 CLASPS-OPPOSITE ARM | 47.28 | 65.84 | 130.59 |
| 2 FRACTURED AREA | 154.84 | 216.87 | |
| 3 CONNECTION SADDLES-MAIN CONNECTOR | 223.87 | 302.93 | 375.88 |
| 4A SADDLE | 48.13 | 77.11 | |
| 4B MAIN CONNECTOR- FRACTURED AREA | 10.99 | 23.74 | 27.73 |
| 5 MAIN CONNECTOR-ANTERIOR AREA | 42.76 | 25.44 | 57.34 |
| 6 MAIN CONNECTOR-POSTERIOR AREA | 8.67 | 13.15 | 26.02 |
| 7 RUSTY CONNECTOR | 207.48 | 203.08 | 307.31 |
| BROKEN CONNECTOR | 165.81 | 176.76 | 179.68 |
| BROKEN CLASPS | 41.17 | 107.83 | 161.5 |
Surface roughness has a considerable influence on fatigue strength because the surface profile resulting from the processing steps is generally composed of numerous stress concentrators (Fig. 3). For initiation in the case of a profile of a specimen with a higher roughness usually a sub-microscopic crack appears at first, which once formed gradually deepens and rapidly this will continuously reduce the strength section of the part and fatigue failure of the element will follow [15].
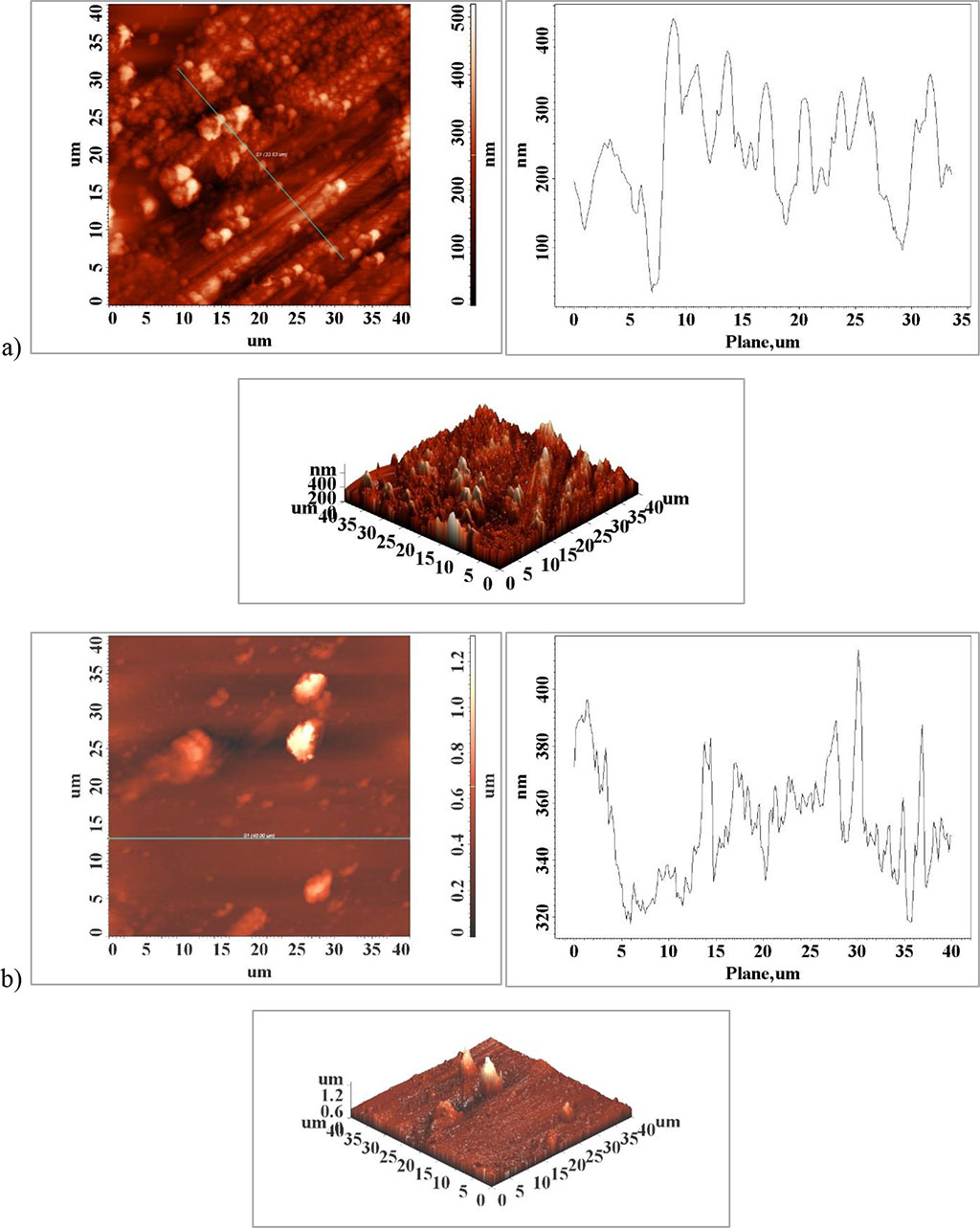
Figure 3. Surface atomic force microscopy (AFM) images of the surface of the clasps element a) unaffected area and b) fractured area
According to the results in the literature by comparing the fatigue limit on different criteria it was revealed that the surface roughness was a significant factor in the material performance and with a high influence on the fatigue strength of the specimen [5]. When the surface roughness (Ra) increases 77 to 176.76 μm, Table 1, Figure 4, a) and d) the fatigue limit of the element will decrease significantly. This can be interpreted as being related to the surface profile whose topography becomes smoother with decreasing surface roughness which will favor the sliding of planes and grains and limit crack initiation at the active element surface. The surface roughness in the fracture zone, Figure 4 c), is much higher than the surfaces in the areas that resisted external stresses, Figure 4 a) and b). In addition to the surface profile of the medical element, some particles of much larger size than the rest of the surface are observed, Figure 4 a) and d), which may be non-metallic in nature, probably corrosion compounds that have appeared on the surface as a result of interaction with the physiological environment.
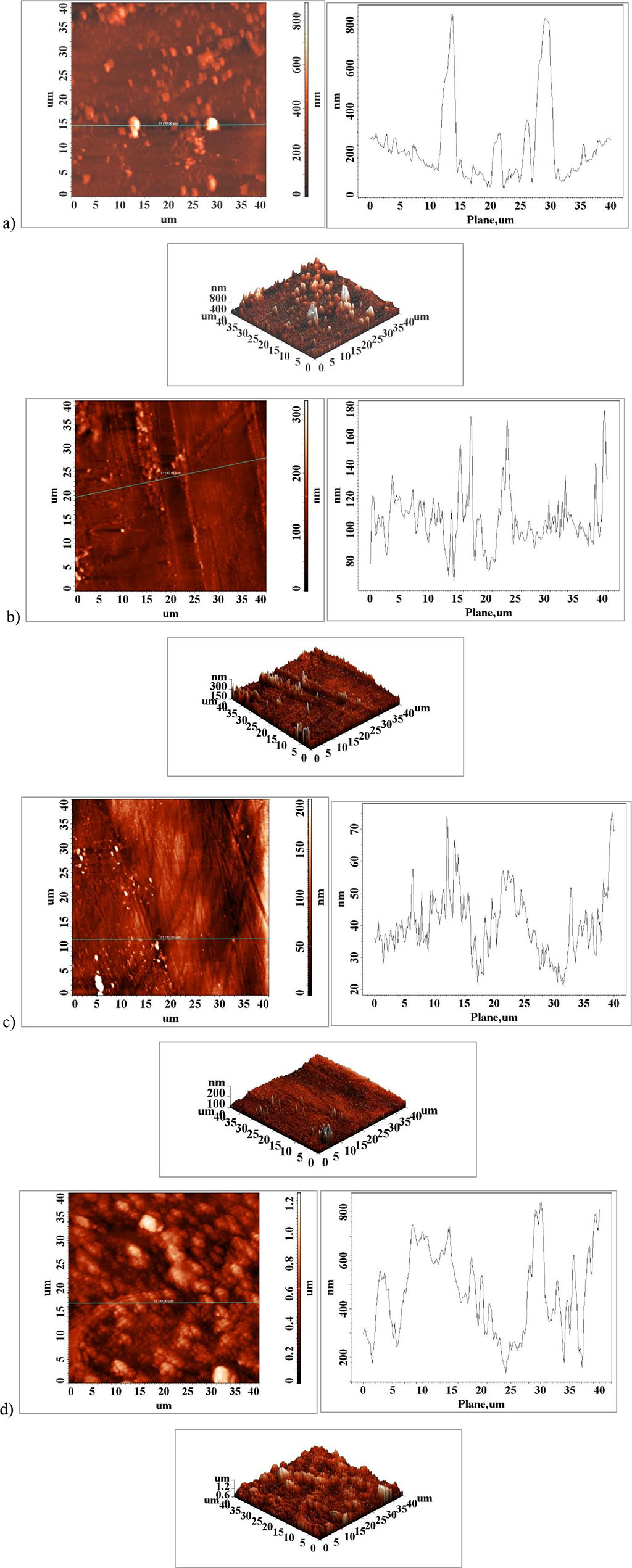
Figure 4. Atomic force microscopy (AFM) surface images of the element surface a) Major Connector- Saddles, b) major connector-anterior area, c) major connector-posterior area, d) fractured major connector
In the case of the fragmented element, no differences in roughness are observed between the surfaces analyzed. The cause of the fracture may be due to incorrect dimensioning and/or design of the element or due to a material defect that led to its failure to external stresses, mechanical fatigue (Figure 5).
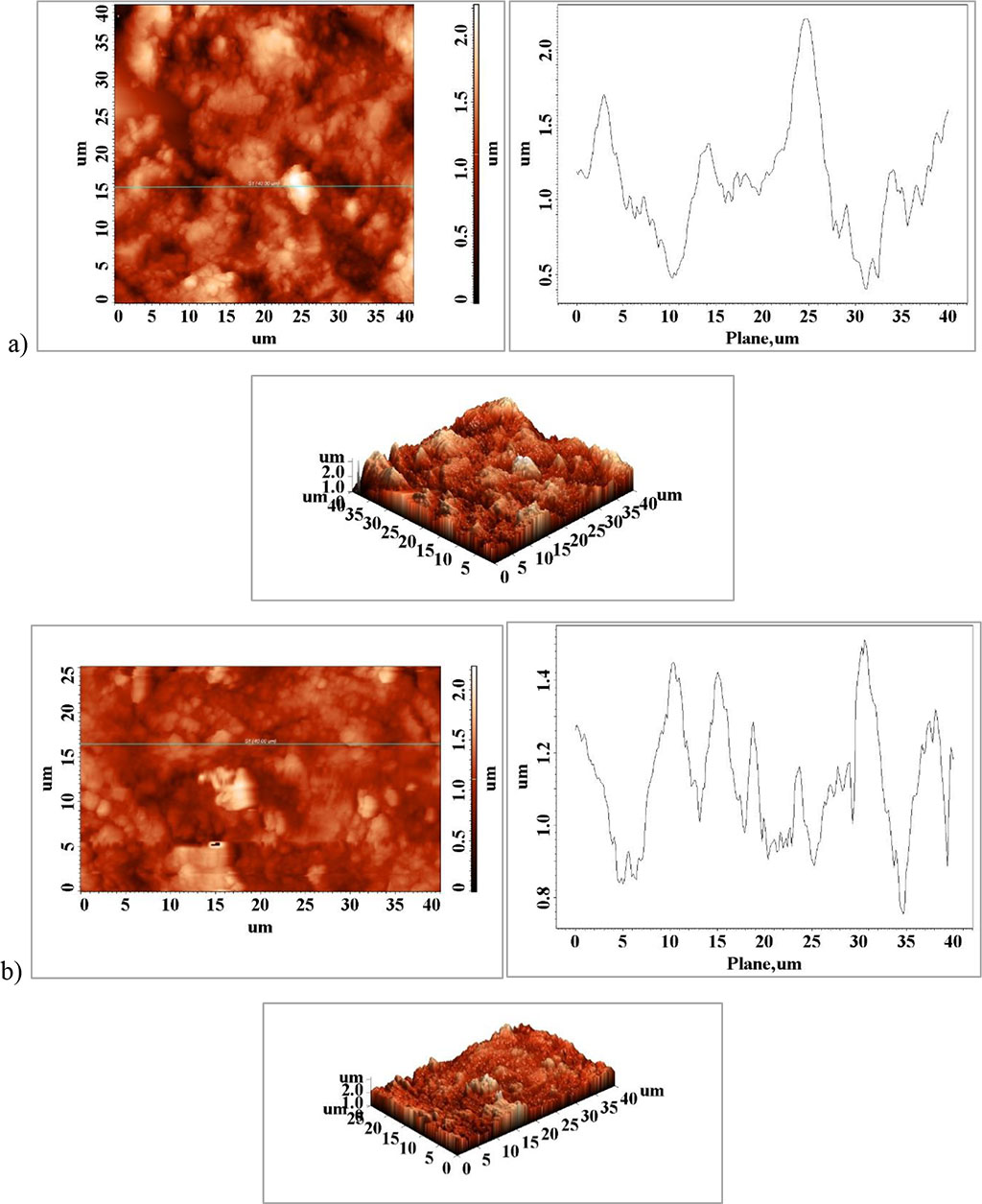
Figure 5. a) fragment connection saddles-major connector, b) fractured fragment
Surface condition is one of the factors that have a definite influence on the fatigue strength of metallic materials. The functionality of metallic or non-metallic elements used in practical applications is to a direct extent dependent on the surface condition of the active elements. For example, if an element has a finished surface with a high roughness, i.e., the fatigue strength decreases greatly, compared to the strength of the same element with a polished surface [5,11]. The fatigue strength of metallic materials in the form of externally stressed elements is influenced by the following factors: surface condition (surface roughness and physical chemical properties of the surface layer); surface heat treatments; surface thermochemical treatments; surface mechanical treatments; metallic coatings; or surface corrosion. The reduction in resistance to repetitive stress under the action of influencing factors is generally judged by a quality coefficient Γ, which by definition is considered to be the fraction between the fatigue strength for a given roughness of the exposed area or in a given condition and the fatigue strength of the same element polished and without surface treatments.
The effect of surface roughness on the fatigue strength of specimens is usually addressed in terms of stress concentration on the surface with higher roughness which will promote stress concentration in certain areas, and which will lead to micro-cracks initiation, bonding and formation of macro-cracks leading to irreversible failure of metallic materials [4,18].
The literature has relatively few in-depth studies related to the fracture causality of skeletal removable partial denture infrastructure; data superimposable on our results are found in the study conducted by Jussara da Silva Barbosa and collaborators, 2021, except that they used fractography as a method to identify specific aspects of fracture zones [9].
Rare fractures at the level of the main connectors may be associated with under sizing in the design of the skeletal partially removable denture, thus a number of parameters were not correctly evaluated at the design stage of the skeletal partially removable denture such as connector cross-section, height, width correlated with antagonist forces, type of prosthetics or natural dentition variant without eluding the type of static and dynamic occlusion.
It is important to note that the fracture of metal components usually targeting vulnerable areas such as the junction areas between the component elements of the skeletal prosthesis or at the level of the brackets may also be due to deficiencies in the strictly technological procedures of preparation for casting or actual turning, technological aspects associated with the classical casting technique.
The surface condition of the active elements used in dentistry influences their fatigue strength to a very large extent. In addition to the surface condition that can promote the appearance of micro-cracks and surface pores, the strength of materials is usually also influenced by the biological environment in which these elements work, an environment that can contribute to the weakening of the strength of materials through the interaction between the biological fluid and the metal material by the formation of corrosion points.
The failure of metallic elements to mechanical-thermal fatigue is not exclusively due to the state of their surface and roughness but may also be due to material or design and dimensioning defects. Usually, the failure of a metal material in operation is due to a combination of factors, which are found in the technological register or may be generated by the particularities of the clinical case that have not been taken into account, so that the individualized version of the partially removable skeletal prosthesis has a balanced biomechanical behavior during the functionality of the masticatory apparatus.
These authors had equal contributions with the first author: Doriana Agop Forna, Nicanor Cimpoesu.