- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
The article discusses the main links of the pathogenesis of allergic contact dermatitis and gives the results of patch testing in patients who were examined to determine the etiological factors of the disease. Screening showed that nickel, cobalt, methylisothiazolinone, phenylenediamine, formaldehydes and fragrances are the most common etiological factors of ACD. Named haptens cause the absolute majority of positive reactions in the selected group of patients, and also cause the largest number of reactions of pronounced intensity. Patch testing is an informative and useful method for a balanced planning in ACD patients treatment strategy, because it reveals the etiological matrix of the pathological process occurrence in the skin.
Keywords: atopic contact dermatitis, haptens, patch testing, nickel, cobalt, etiological matrix.
Allergic contact dermatitis (ACD) is a delayed-type hypersensitivity reaction (type 4 according to the Gell-Coombs classification). The initial or induction phase of ACD occurs when an allergen (hapten) combines with a protein, forming a complex that leads to the development of an allergen-specific population of T cells. Repeated exposure to the antigen causes an immune response mediated by T cells and leads to the development of allergic contact dermatitis. ACD accounts for up to 20% of contact dermatoses, and allergens vary widely by geography, personal habits, occupations and hobbies, and often by the types of preservatives that are legally permitted. In particular, quaternium-15 is permitted in the United States but banned in Europe [1-4].
The morphology and localization of contact dermatitis often immediately indicate an allergen. For example, the appearance of an inflammatory rash around the wrist may indicate an allergic reaction to a bracelet or watch strap. Nickel is one of the most common causes of AKD and can be manifested by wearing chains, earrings, rings, belts with metal buckles. Other most common allergens include preservatives, fragrances, textile chemicals, and sunscreens [4].
The pathophysiology of allergic contact dermatitis begins with contact of the allergen with the skin. This allergen penetrates the stratum corneum of the skin and contacts Langerhans cells [5-8]. Langerhans cells then migrate to regional lymph nodes and present captured antigens. Thanks to the process of cytokine-induced proliferation, antigen-specific T-lymphocytes are created. These lymphocytes can then enter the epidermis through the blood. This process is known as the sensitization phase of allergic contact dermatitis (Fig. 1).
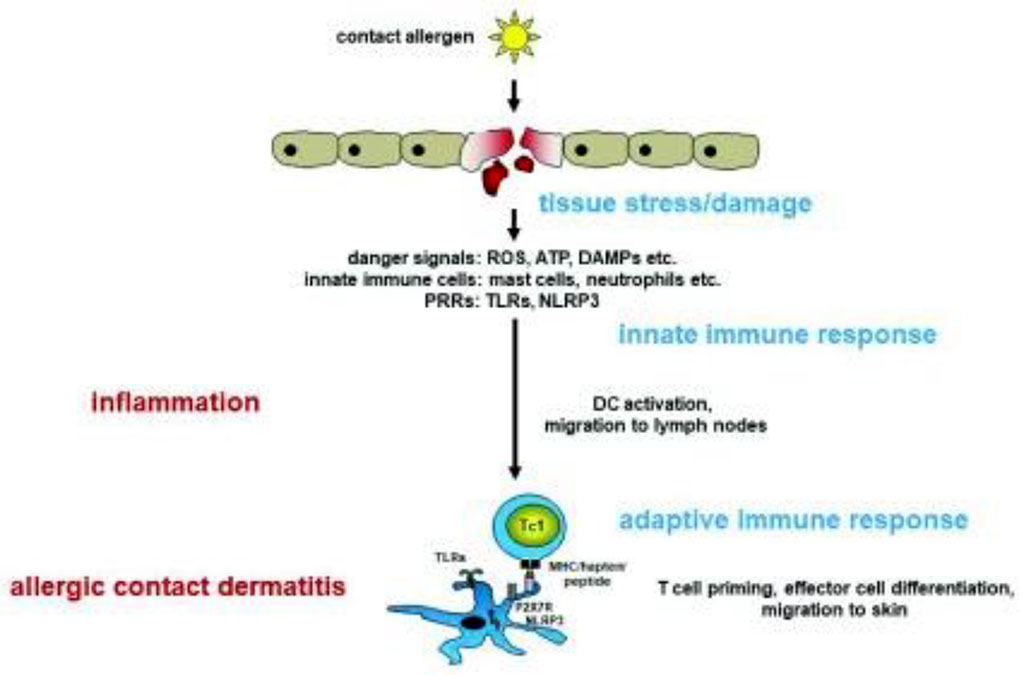
Figure 1. The sensitization phase of allergic contact dermatitis /Martin SF, 2018/.
The exposure phase is what happens after repeated exposure to the antigen. Antigen-containing Langerhans cells interact with antigen-specific T lymphocytes for that antigen, which triggers a cytokine-induced proliferation process. This proliferation, in turn, creates a localized inflammatory response (Fig. 2).
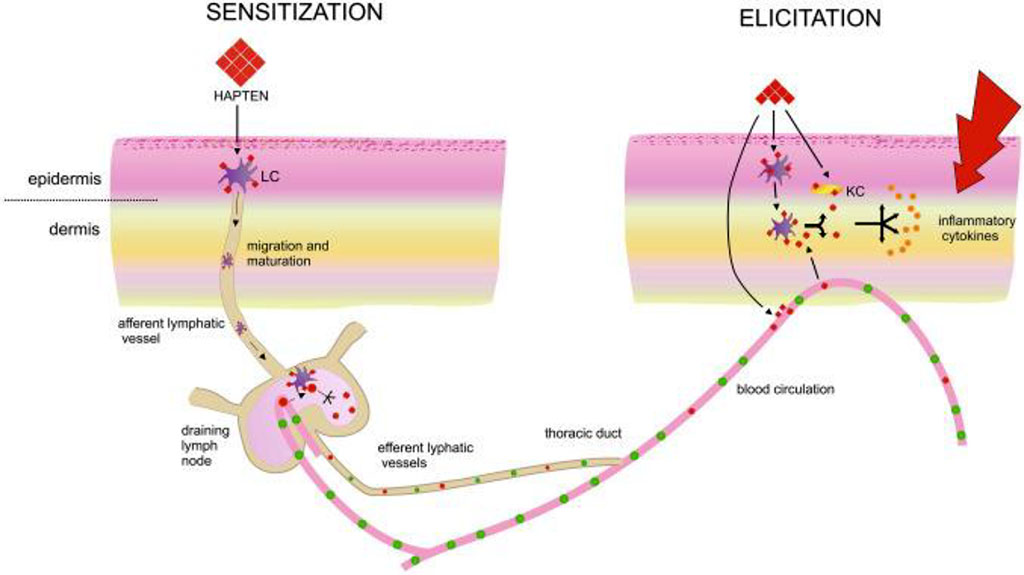
Figure 2. The exposure phase of allergic contact dermatitis /Kiecka A, 2023/.
Typical symptoms include: hyperemia, often without clear boundaries, but usually starting at the site of contact, swelling, scaling, itching. At first, the symptoms affect only the part of the body that comes into contact with the allergen. Most often, these are the hands, especially the fingers and the back of the palm. Other areas commonly affected include the face (especially the eyelids and lips), neck, lower legs, and feet. The severity of the skin allergic reaction mainly depends on the substance with which the skin was in contact and for how long [9,10]. With allergic contact dermatitis, allergic manifestations may also occur later on other areas that have not been in contact with the allergen (hapten).
The main and mandatory component of the treatment of ACD is the identification and avoidance of contact with the allergen (hapten). First-line drug therapy includes topical steroids if ACD is limited to less than 20% of the body and oral corticosteroids if more than 20% of the body is involved. If the process involves a delicate area, such as skin folds or eyelids, topical calcineurin inhibitors may also be effective [12]. The use of moisturizers is a recommended addition to care.
In cases of chronic or persistent ACD, patch tests should be used to identify the allergen [13]. Successful patch testing requires several components: selection of an appropriate panel to test, a positive patch test for the appropriate allergens, and counseling of the patient regarding the patch test results. Additionally, the American Contact Dermatitis Society's Contact Allergen Management Program (CAMP) can be used to create a "safe list" of products that do not contain the patient's allergens. In the event that allergens cannot be avoided, systemic therapy may be required [14]. Patch-testing is used to obtain a miniature eczematous reaction by applying allergens under occlusion to the intact skin of patients with suspected ACD.
The most important part of patch testing is patient education, which occurs after the results are finally read. It is important to provide the patient with written information about all allergens with a positive reaction. This written information should include the name of the allergen, synonyms, typical uses, and strategies for avoiding the chemical [15].
Objective: To determine which allergens most often cause ACD in the studied group and to develop recommendations for personalized diagnosis and treatment of the specified pathology.
The study included 112 patients, among them 81 women and 31 men, with clinical manifestations of ACD, who had a confirmed diagnosis using patch testing (European panel S-1000e). Treatment of patients was carried out according to the protocols and in accordance with the recommendations of the Ministry of Health of Ukraine.
The 30 most common hapten tests were included in the punch test panel: Potassium dichromate, Phenylenediamine, Thiuram mix, Neomycin sulfate, Cobalt chloride, Benzocaine, Nickel, Clioquinol, Colophonium, Paraben, N-isopropil-N-phenyl-4phenylenediamin, Lanolin alcohol, Mercapto mix, Epoxyresin bisphenol, Peru balsam, 4-tert-butylphenolformaldehyde resin, Mercaptobenzothiazole, Formaldehyde, Fragrance mix, Sesquiterpene lactone mix, Quaternium, Methoxy-6-n-pentyl-4-benzoquinone, Methylchloroisothiazolinone, Budesonid, Tixocortol-21-pivalate, Methyldibromoglutaronitrile, Methylisothiazolinone, Fragrance mix II, Hydroxyisohexyl 3-cyclohexenecarboxaldehyde, Textile dye mix.
The test system also provided a qualitative assessment of the severity of the reaction to haptens: "***" - pronounced reaction, "**" - moderately expressed, "*" - weakly expressed.
The study found that patients responded differently to the proposed haptens in a punch-testing panel. Patients did not react at all to 10 out of 30 haptens of the European series, these were tests for Neomycin sulfate, N-isopropyl-N-phenyl-4phenylenediamin, Mercapto mix, Epoxyresin bisphenol, Mercaptobenzothiazole, Sesquiterpene lactone mix, Methoxy-6-n-pentyl-4 -benzoquinone, Tixocortol-21-pivalate, Fragrance mix II, Hydroxyisohexyl 3-cyclohexenecarboxaldehyde. Also, the study revealed that many patients showed a positive reaction to several haptens. The study demonstrated that in 38 tests for 7 haptens, a pronounced reaction was noted, the manifestation of which was most often in response to nickel; in 86 tests for 18 haptens a moderately expressed reaction was observed and in 139 tests for 18 haptens a weakly expressed reaction was observed (Table 1).
Table 1. The list of haptens and the number of patients with different severity of reaction to them in the study group
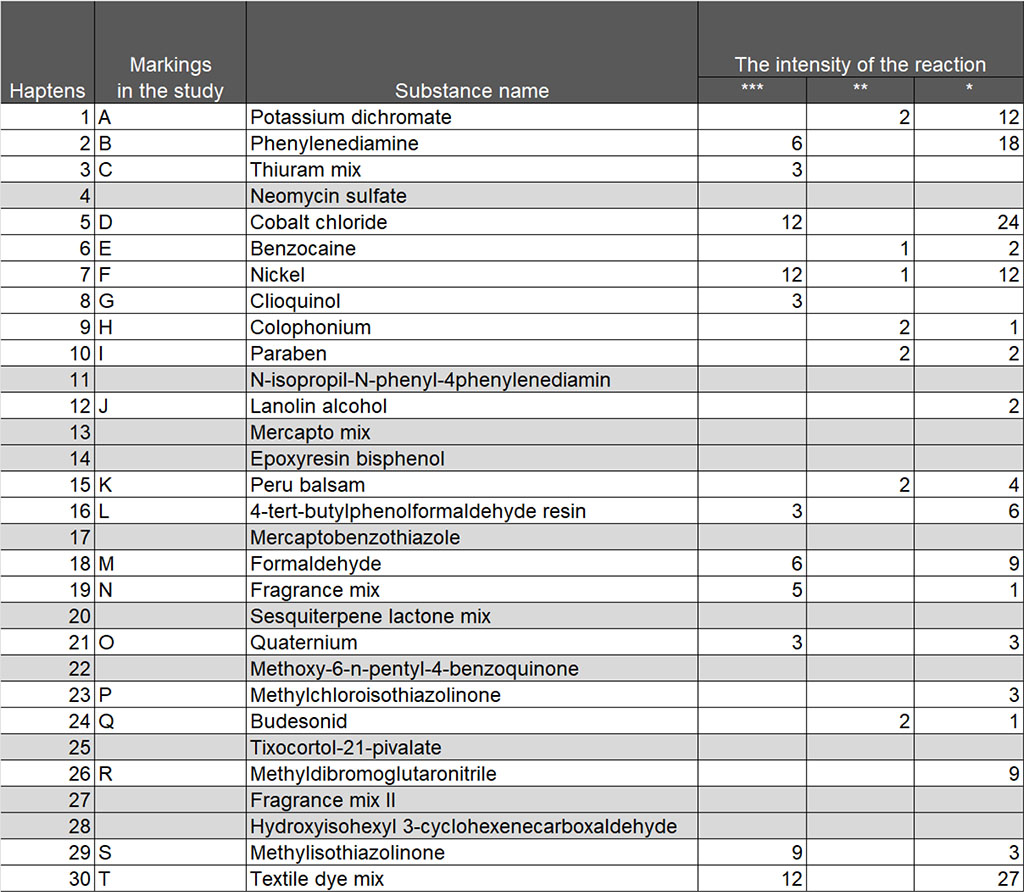
In total, positive test reactions were recorded for 20 haptens, and to present the results in diagrams, it was suggested to give allergens the appropriate designations with letters of the English alphabet: A - Potassium dichromate, B - Phenylenediamine, C – Thiuram mix, D - Cobalt chloride, E - Benzocaine, F - Nickel, G - Clioquinol, H - Colophonium, I - Paraben, J -Lanolin alcohol, K - Peru balsam, L - 4-tert-butylphenolformaldehyde resin, M -Formaldehyde, N -Fragrance mix, O -Quaternium, P -Methylchloroisothiazolinone, Q -Budesonid, R - Methyldibromoglutaronitrile, S - Methylisothiazolinone, T - Textile dye mix.
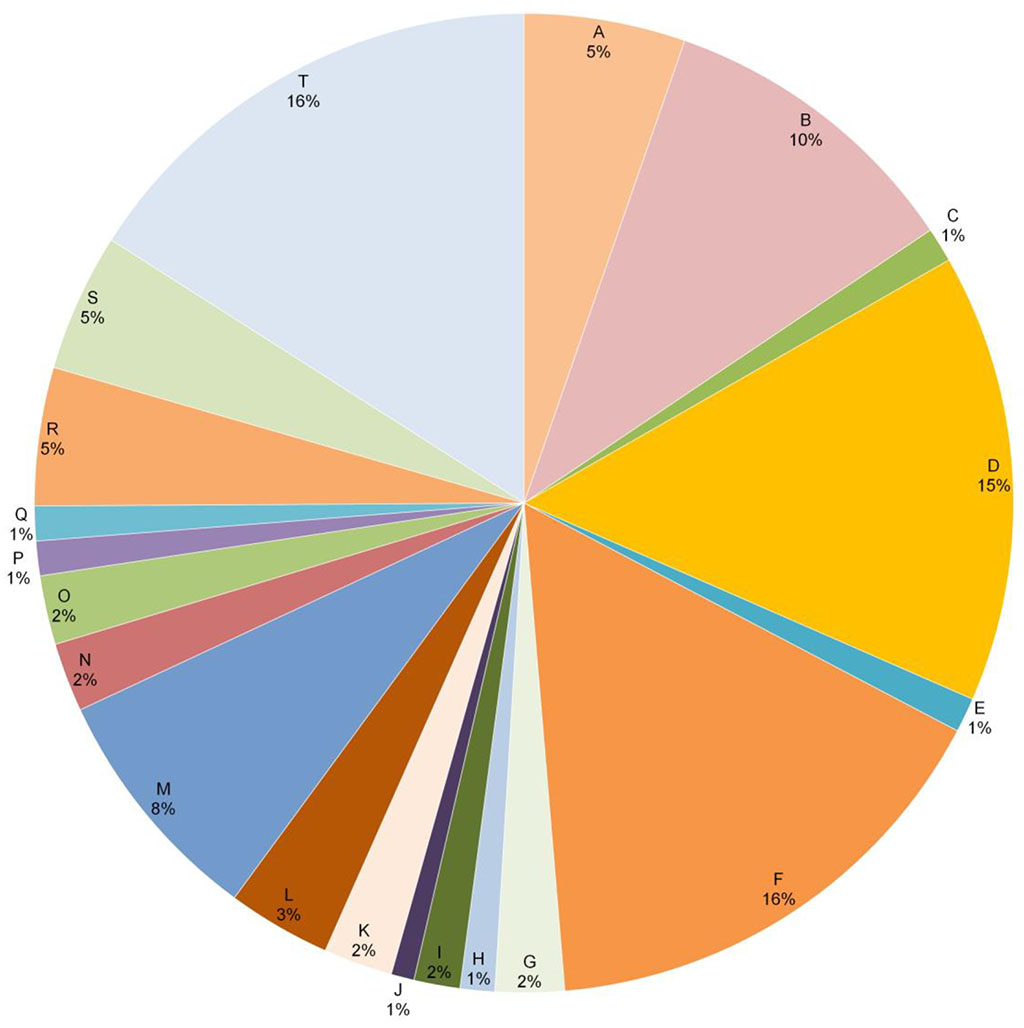
Figure
3. Haptens to which patients responded and their share in the study
group, %.
A
- Potassium
dichromate,
B - Phenylenediamine, C – Thiuram
mix,
D - Cobalt
chloride, E - Benzocaine, F
- Nickel, G
- Clioquinol, H
- Colophonium, I
- Paraben,J
-
Lanolin
alcohol,
K - Peru
balsam, L - 4-tert-butylphenolformaldehyde
resin,
M -
Formaldehyde, N -
Fragrance
mix, O -
Quaternium, P -
Methylchloroisothiazolinone, Q -
Budesonid, R - Methyldibromoglutaronitrile,
S - Methylisothiazolinone,
T - Textile
dye
mix.
As can be seen from Figure 3, the largest share of positive reactions is attributed to nickel (16%), textile dyes (16%), cobalt (15%), phenyldiamine (10%) and formaldehyde (8%). Potassium dichromate, methyl-dibromo-glutaronitrile and methylisothiazolinone were distributed among 5% of positive tests, the rest had a lower specific gravity.
At the same time, it should be noted that nickel (47%) and formaldehyde (16%) prevailed among those haptens to which a pronounced positive reaction was observed; the rest was textile dye mix (8%) and methyl-dibromo-glutaronitrile (5%) (Fig. 4).
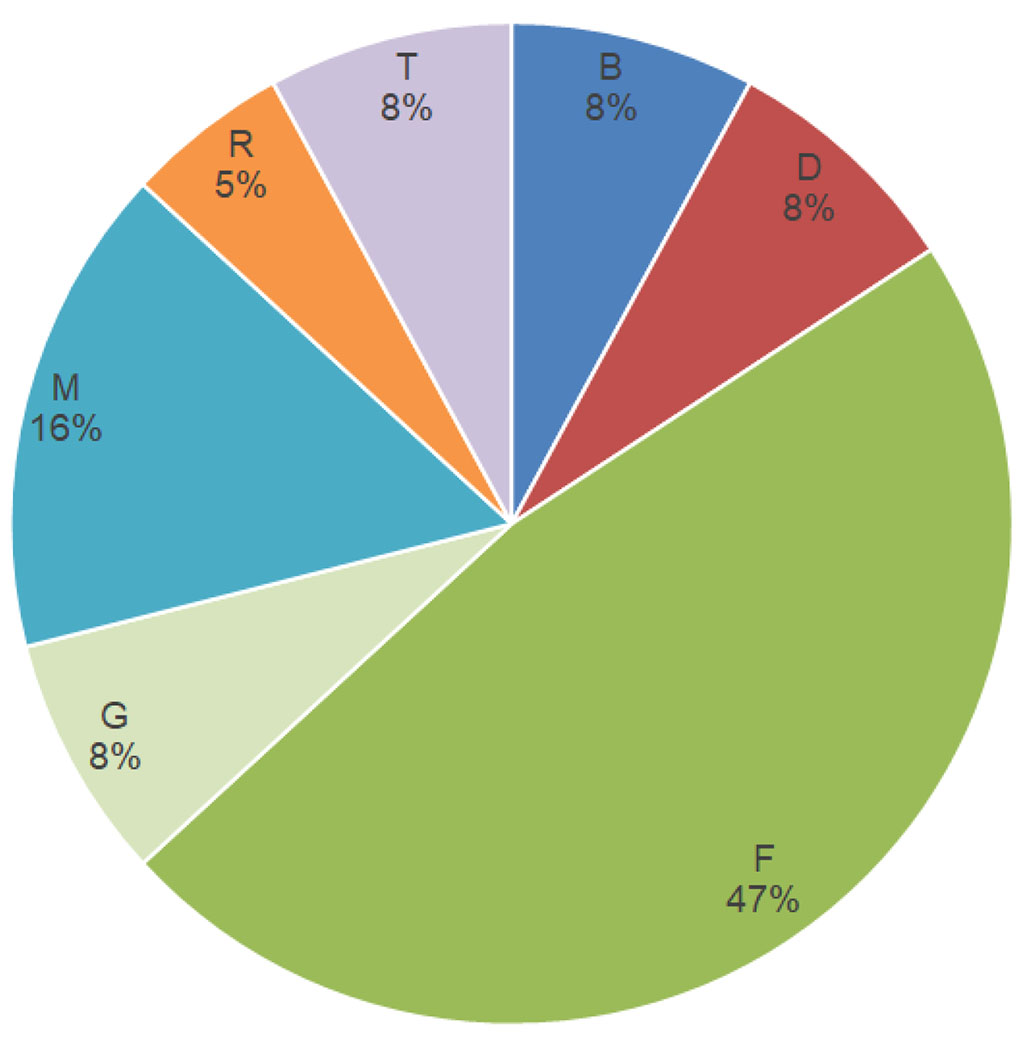
Figure
4. Haptens to
which the most pronounced reaction was observed (***).
B
- Phenylenediamine, D - Cobalt
chloride, F
- Nickel, G
- Clioquinol, M -
Formaldehyde, R – Methyldibromoglutaronitrile,,
T - Textile
dye
mix.
A reaction of medium intensity was recorded mainly to cobalt (14%), nickel (14%) and a mix of textile dyes (14%), as well as to methylisothiazolinone (10%) and formaldehyde (7%), the proportion of reactions to other haptens was much smaller ( Fig. 5). A weak reaction was also observed in response to certain allergens, but it should be noted that the etiological factors of the vast majority of such reactions were phenylenediamine, cobalt and textile dyes, as can be seen from Table 1.
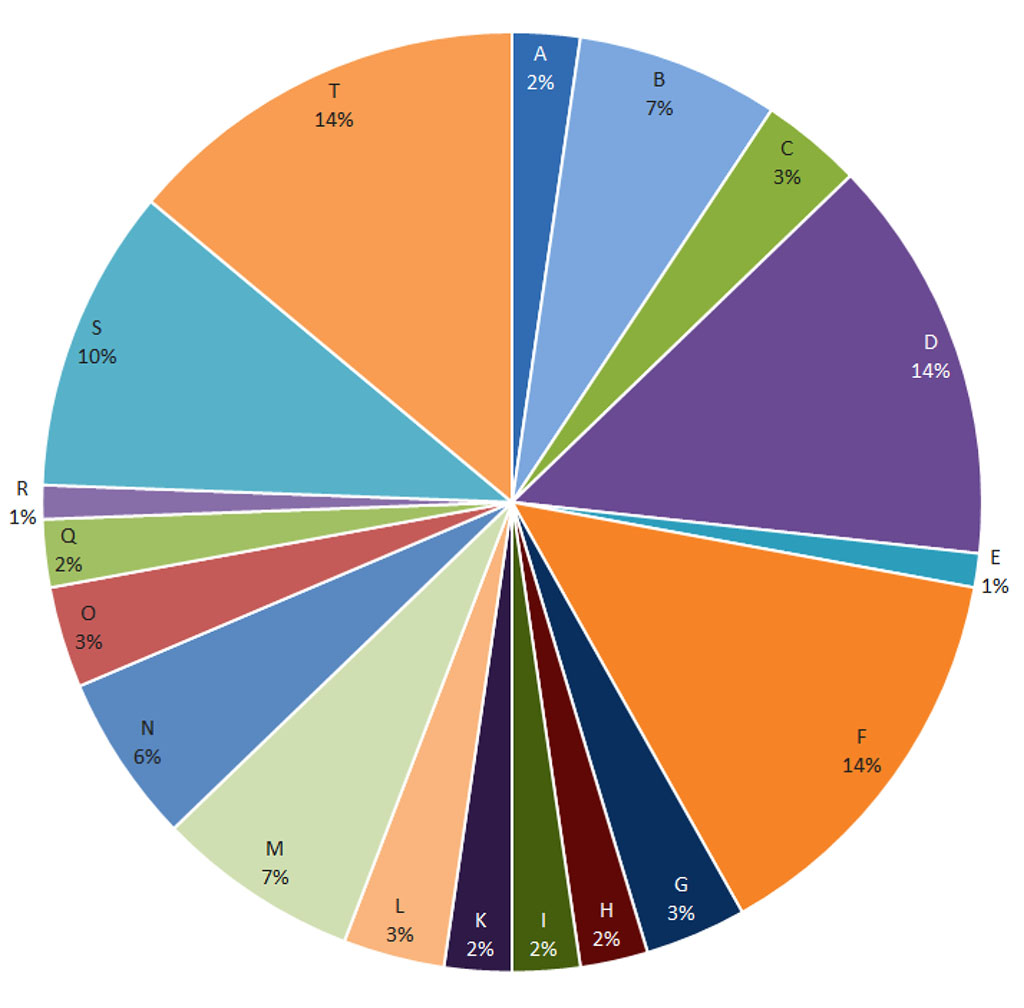
Figure
5. Haptens to which a moderate reaction was observed (**).
A
- Potassium
dichromate,
B - Phenylenediamine, C – Thiuram
mix,
D - Cobalt
chloride, E - Benzocaine, F
- Nickel, G
- Clioquinol, H
- Colophonium, I
- Paraben,
K - Peru
balsam,
L - 4-tert-butylphenolformaldehyde
resin,
M -
Formaldehyde, N -
Fragrance
mix, O -
Quaternium,
Q -
Budesonid, R - Methyldibromoglutaronitrile,
S - Methylisothiazolinone,
T - Textile
dye
mix.
The results of the conducted research allow us to consider nickel, cobalt, textile dyes, methylisothiazolinone, phenylenediamine and formaldehyde as haptens that prevail in the selected cohort of patients.
It is well known that about 20% of the population has a contact sensitivity to common haptens such as fragrances, preservatives and metals. Many also develop allergic contact dermatitis as a clinical manifestation of contact sensitization. ACD is a public health problem and one of the most important occupational diseases, which is mediated mainly by memory T lymphocytes, which recognize low molecular weight chemicals after skin contact [5].
The results of our study complement the data of the research literature, from which it is known that nickel, cobalt, methylisothiazolinone, phenylenediamine, formaldehydes and fragrances are the most common etiological factors of ACD [8,16]. In addition, it is these actual haptens that cause the largest number of pronounced reactions, which is confirmed by the results of patch testing. The above-mentioned haptens also cause the absolute majority of positive reactions in the selected group of patients.
Of interest is the fact that the presence of positive reactions to several haptens in different patients does not affect the severity of the allergic reaction, that is, adaptive mechanisms during inflammation are able to compensate for the simultaneous effects of various allergic factors. Conversely, even one hapten, in the absence of positive tests for other haptens, can cause a pronounced reaction in certain categories of patients [9,17].
Screening showed that the etiological matrix of ACD can be diverse and unpredictable, but still it is built according to certain laws, in particular if we take into account reactivity to the most studied allergens and pay attention to the intensity of inflammatory skin changes in response to allergic exposure. Research in this direction must necessarily continue.