- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cells of non-hereditary retinblastoma in the enucleated eye of a 9-year-old boy were studied using classical histological and immunohistochemical methods. It has been established that disturbances in retinal morphogenesis can be associated with a stop in development at the stage of formation of retinal layers. The inner wall of the eyecup after the eviction of the neuroglia retained proliferative activity in the cells of the outer retinal layer, which lost the ability to differentiate over a larger extent of the retina and migrate to the inner layers. Cells that have differentiated according to the program of neuronal development are located among malignant cells and are characterized by signs of atypical aging with granules of black-brown pigment in the cytoplasm. It has been established that in the lack of angiogenesis these cells in the inner layers of the retina have secretory activity and are immunohistochemically phenotyped as VEGF-positive cells. This indicates cellular and tissue adaptation to malignancy at the terminal stage of retinoblastoma, which grows into the optic nerve and metastasizes to the brain. VEGF-positive cells can serve as one of the key targets in solving the problem of conservative treatment of retinoblastoma diagnosed in the late stages of the disease.
Keywords: neuro-oncology; pediatric oncology; retinoblastoma; aging of neurons; eye.
Analysis of scientific literature since 1931, in particularly, the works by Ancona-Lezama D., Dalvin L.A., Shields C.L. (2020) shows that the treatment of retinoblastoma has remained complex, requiring individualized treatment based on staging of the International Classification of Retinoblastoma (ICRB) [1]. According to Fabian I.D., Onadim Z., Karaa E., and co-authors (2018), retinoblastoma in children is registered in one case among 15,000-20,000 newborns [2]. According to Mendoza P.R., Grossniklaus H.E. (2015) in the pathogenesis of RB lies a loss of function mutation in the RB1 gene on chromosome 13q. The pathology is associated with a defect in the RB1 gene in the cases of both a hereditary disease and if not confirmed in the family history. [3]. Dasgupta P., Padmanabhan J., Chellappan S. (2006) in their studies found that the RP gene affects retinal cells differently, they react differently, modulating various cellular phenomena, including proliferation, which are more studied; as well as a resting state, differentiation, senescence, and apoptosis, which are less studied [4]. A question of the conditions for genome inhibition and the formation of a spectrum of repressive and inducible genes in retinal cells needs to be solved. However, the secreted signaling molecules which control eye morphogenesis and are essential for visual functions have not been studied enough. Knudson A.G. Jr. (1971) hypothesized that retinoblastoma is caused by two mutational events. In the dominantly inherited form, one mutation is inherited through germ cells, and the second occurs in somatic cells [5]. Currently, the origin of retinoblastoma cells has been identified. RB cells originate from differentiating cone precursors or from earlier precursors of neurons and neuroglia – spongioblasts with possible conditions for their reprogramming in case of genomic disorders.[6]. Norrie J.L., Nityanandam A., Lai K., et al. (2021) tried to study the mechanism of RP in a mouse model after transplantation of tumor cells obtained from the human eye [7], however, such extrapolation can only be conditional. Zheng C., Schneider J.W., Hsieh J. (2020) believe that although mouse models of retinoblastoma have some similarities with human retinoblastoma, there are differences in their cellular differentiation [8]. The development of conservative methods for the treatment of retinoblastoma with preservation of the visual functions of the cancerous eye instead of enucleation requires understanding of the pathogenesis of retinal malignancy. The insufficiency of research data on intercellular interactions in the morphogenesis of the retina, underlying the pathogenesis of retinoblastoma, has motivated the choice of our research.
The aim of our work is to study the features of cell phenotypes in the structure of terminal retinoblastoma.
Materials of a clinical case of retinoblastoma in a 9-year-old boy were used in this work. The patient's eye, enucleated at the last stage of retinoblastoma, affected by malignancy, with growth in the optic nerve and metastases in the brain, after extirpation, was processed in accordance with the protocols for classical staining with hematoxylin and eosin and performing immunohistochemistry to detect VEGF-positive cells. The illustrative material was obtained and analyzed using an OLYPUS BX51 microscope with a DP25 camera and a proprietary software application for morphological studies.
The general picture of retinoblastoma is characterized by depigmentation of the pigment epithelium and retinal detachment, represented by tumor cells, among which glial cells with a light chromophobic cytoplasm are identified in a small number (Figure 1). The pigment epithelium is located on a thickened basement membrane, which may border on germline neuroglia.
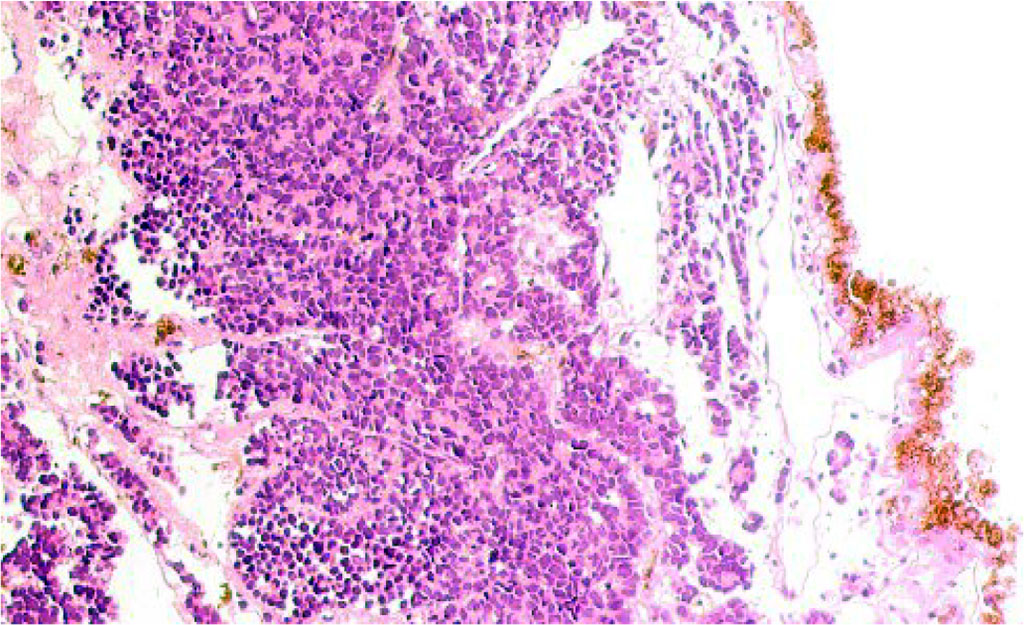
Figure 1 - Retina of a 9-year-old boy with retinoblastoma. Microphoto. Stained with hematoxylin and eosin. Magnification x100.
It has been established that retinoblastoma is accompanied by increased accelerated aging of neurons. In the retinal structure, against the background of tumor cells, differentiated neurons are identified, containing a large number of brown granules in the cytoplasm. I.T. Niculescu (1963) detected brown and black granules in the cytoplasm of neurons, which retained hyperpigmentation after staining with hematoxylin and eosin, are evidence of neuronal aging against the background of a decrease in oxidases [9]. The composition of the black-brown pigment includes lipoid and melanin components in the form of a mixture of lipofuscin and melanin, resulting from the action of an autolytic enzyme and tyrosinase. An increase in the amount of pigment correlates with a decrease in cellular oxidation processes. There are two points of view on the accumulation of pigment by neurons. According to the first, this process reflects the early and atypical old age of neuronal structures; according to the second, this process develops as an adaptation to ischemia and hypoxia. In the central nervous system, pigment-containing neurons belong to the autonomic nervous system and are absent in short-lived animals. Sizes of neurons vary from 12 to 100 micrometers (Figure 2a), and the majority of them have a spindle-shaped form (Figure 2 b, c). Cells with a ring-shaped arrangement of chromatin in the nuclei are identified, with signs of apoptosis (Figure 2d).
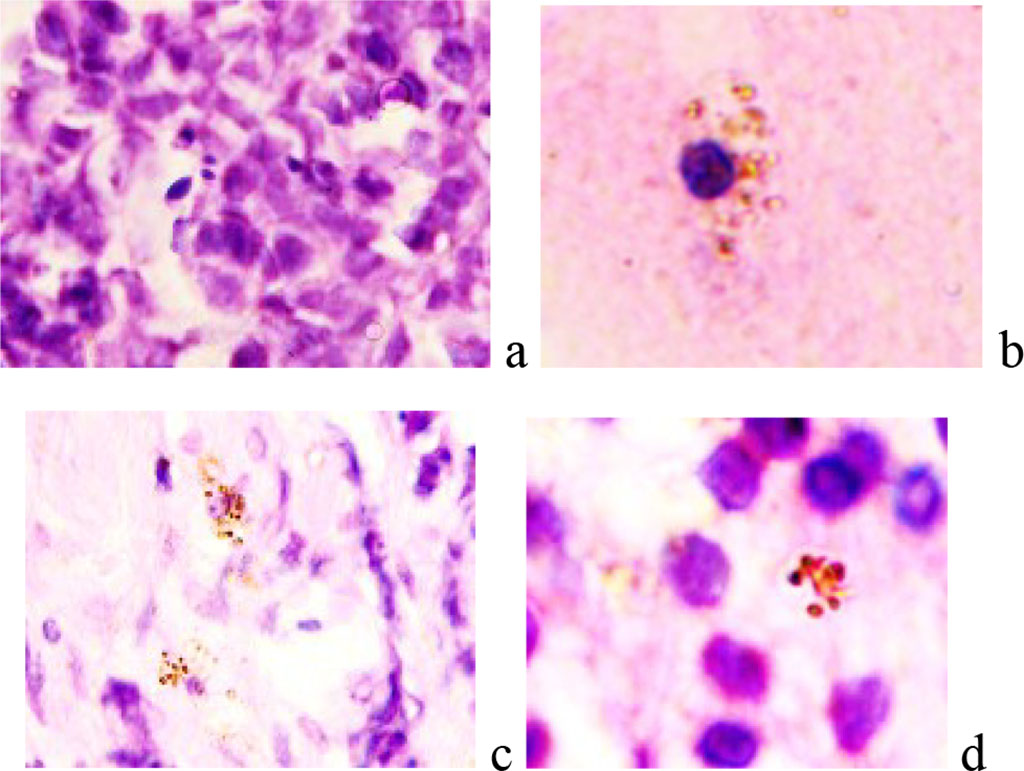
Figure 2 - Retina of a 9-year-old boy with retinoblastoma. Microphoto. Stained with hematoxylin and eosin. Magnification x400.
Among the blast cells of RB, giant cells of astrocytic neuroglia are identified. It has been established that there are no blood vessels in the bipolar and ganglionic layers of the retina. At the same time, VEGF - positive cells of a rounded shape are identified in the avascular layers of the retina. Endothelial growth factor activity is especially pronounced around the nucleus and can appear as a semicircle at one of the cell poles (Figure 3 a, b, c, d, f. g). Uniform expression is observed in large spindle-shaped cells, sometimes extending to one of the processes (Figure 3 k, l).
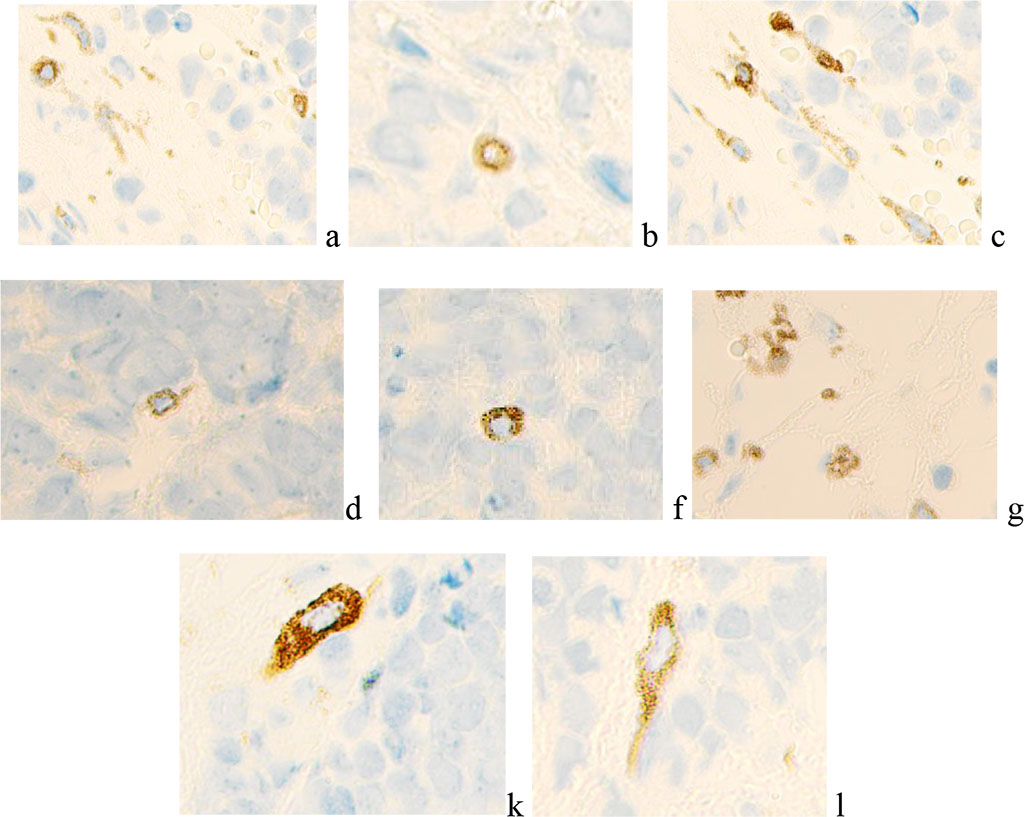
Figure 3 - Microphoto. Immunohistochemistry to detect the localization of VEGF-positive cells. Nucleus staining with hematoxylin. Magnification x400.
The presence of VEGF-positive cells in the retina of an eye affected by retinoblastoma, in the setting of multiple senescent cells containing pyment granules, in the absence of blood vessels, indicates tissue adaptation to hypoxia and induction of VEGF secretion.
In the development of the retina, hypoxia plays an important role in the inhibition of proliferative processes, the onset of differentiation, and the formation of interneuronal retinal connections. There are no blood vessels in the liquor-independent layers of the retina under conditions of malignancy; adaptation to hypoxia is realized due to VEGF-positive cells, as well as due to the accumulation of aging pigment, which simultaneously acts as an oxygen depot and positively correlates with the level of oxidases. The question of why some neurons differentiate and even age, and whether these cells are similar to the autonomic neurons of the central nervous system, which are also capable of accumulating pigment, needs to be studied. The obtained data complement the knowledge about the mechanisms of retinoblastoma and provide insight into promising cellular targets in the development of targeted conservative therapy for retinoblastoma. Further study and search for signaling pathways that inhibit cell differentiation into retinal neurons and neuroglia is required.