- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
In solid malignant tumors, as well as in hematological tumors, cancer stem cells (CSCs), quiescent, pluripotent, self-renewing neoplastic, also known as tumor initiating cells, have been identified. CSC tumors are resistant not only to chemotherapy, but also to radiation therapy, as well as they play their role in the occurrence of relapses after surgical treatment, An unfavorable prognosis due to an aggressive course and complications after treatment of the most common primary brain tumor - glioblastoma multiforme (GBM), is also associated with CSC.
One of the mechanisms that affect the physiological regeneration of tissues, but do not work under conditions of malignancy, is the environment and composition of the cellular tumor ensemble, represented by the phenotypes of cellular and adaptive immunity. Using immunohistochemical phenotyping methods, the authors identified immunocytes/phagocytes and established the features of their localization in the malignant tissue and at the border around the tumor. It was concluded that due to apoptosis around the tumor, intercellular signaling and migration of immunocytes/phagocytes to the focus of the tumor process are disrupted. The primary tumor process is not associated with damage to the tissue cambium genome, but with the migration of immature stem cells of the hematogenous pool, capable of proliferating and partially entering into incomplete differentiation under conditions of hypoxia and the absence of blood vessels. At the second stage, connective tissue is formed, which grows into vessels, the wall of which is represented by immature endothelium, with impaired transport function. According to the authors, metastasis of cancer cells does not occur by hematogenous and lymphogenous pathways, but is associated with the development of additional foci of hematopoiesis as a result of the adaptation of the human body to the loss of stem and semi-stem cells, precursors for normal regeneration of the nervous tissue.
Keywords: brain cancer; endothelial cells; cancer stem cells; glioblastoma; glioma stem cells, glioma cellular phenotypes; functional analysis; tumor resection.
At the present stage, information about the significance of cancer stem cells in the pathogenesis and resistance to treatment of glioblastoma is insufficient [1, 2, 3]. We know already there are mechanisms of induction of disturbances in local differentiation of glioma stem cells (GSCs) associated with the microenvironment of tumor cell ensembles, the concept of niches, metabolism, impaired antigen presentation and control of proliferation and cell differentiation of CSCs in the area of malignancy by immunocytes, damage to the genome and the influence of the epigenome. However, they do not provide an exhaustive answer about signaling intercellular interactions under physiological regeneration of the nervous tissue of the brain that affect the differentiation of CSCs, which are a promising target for targeted cancer treatment [4, 5]. Currently, the question for the strategy of targeted treatment of primary and secondary glioblastomas, which have different key progenitor cells in their structure targeted with a different response to therapeutic measures, remains open. According to Sun Q., Chen Y., Liang C., Zhao Y., Lv X., Zou Y., Yan K., Zheng H., Liang D., Li Z.C. (2021) the biological significance of prognostic tumor phenotypes remains poorly understood, which affects the choice of pathogenetically based and dosed treatment [6].
Despite the histological external identity, primary and secondary glioblastomas differ in genetic and epigenetic profiles, since the latter are identified by IDH1 mutations, and indicates the origin of the tumor from the precursors of nerve cells, in contrast to the primary ones, which are of glial origin and are characterized by a higher degree of malignancy. There is no solution to the issue of resistance of tumor cells to anticancer treatment [7]. Considering the role of chemokines and cytokines in intercellular interactions in the tumor structure with suppression of the adaptive immune response, inhibition of the immune response aimed at regulating cell differentiation and tumor involution, there is an urgent need to study the spectrum of cellular ensembles of immunocytes/phagocytes, proliferating, apoptotic and differentiating cells in the tumor structure. and in her environment. The controversial ideas of the existing concepts of carcinogenesis, the lack of comprehensive data on cellular phenotypes in brain tumors determined the direction of our research.
The aim of our work was to study cellular phenotypes of brain tumors in patients of Primorsky Krai (Russia) operated on for primary, secondary and recurrent tumors.
The research obtained permission of the Ethical Committee of School of Biomedicine (Far Eastern Federal University, Vladivostok, Russia), in accordance with the Declaration of Helsinki 2000 and additions of 2013. Based on clinical indications and with the informed consent of patients, biopsy specimens of primary and recurrent brain tumors were taken. The patients were distributed by age, sex, tumor localization, number of samples taken (Table 1).
Table 1. Distribution of material by age, sex, tumor localization
| No | Age (years) | Floor (m/f) | Biopsy, number of samples | primary secondary | Clinical diagnosis, tumor localization |
| 1 | 4-10 | 2/2 | 14 (4/12) | primary | Volumetric formations of the ventricles of the brain from 2 sides |
| 2 | 11-15 | 5/4 | 15 (8/7) | primary | Medulloblastoma |
| 3 | 16-25 | 4/5 | 14 (9/5) | primary | Tumor of the brain |
| 4 | 26-45 | 9/1 | 29(18/11) | secondary | Tumor of the brain |
| 5 | 46-60 | 7/3 | 16 (7|9) | Secondary relapse | Tumor of the brain, spinal cord tumor |
| 6 | 61-68 | 2/3 | 18 (11/7) | Secondary, relapse | frontal lobe tumor |
| Total | 47 (29/18) | 92 (53/39) | |||
* Note. Absolute numbers used
Using immunohistochemical methods, we analyzed the material of 92 biopsies of brain tumors of patients aged 4 to 68 years. The biopsies were obtained from 47 patients who underwent brain tumor surgeries for glioblastomas of different locations in the Department of Neurosurgery of our Medical Center since January 2015 to June 2021. In accordance with the classical protocols of the supplier of immunohistochemical reagents for light microscopy Dako, the activity of the Ki67, p53 and p63 genes was studied to identify the ratio of the level of proliferation and apoptosis of structural elements in the malignant nervous tissue, and the phenotypes of immunocytes/phagocytes CD4, CD8, CD68, CD163 in brain tumors and their environment. The analysis of the results was carried out using an Olympus BX51 microscope, illustrations were obtained using a DP x25 digital camera, statistical processing of the material under study was carried out using proprietary computer programs from Olympus (Japan).
By detecting the protein of the Ki67 gene, the degree of proliferative activity of tumor cells was established. A conclusion was made about the correspondence and general patterns of malignancy of the nervous tissue in comparison with other tissues. Apoptosis of nerve cells and neuroglia was noted against the background of proliferation of malignant cells. The resulting archive of material showed age-related dynamics and features of oncological pathology of the brain in patients of Primorsky region. We noted that the tumors were characterized by both abundant blood supply and were weakly vascularized, could have clear boundaries and show signs of invasive growth. The pathomorphological picture of most neoplasms corresponded to gliomas, which is within the framework of the global statistics of brain tumors. In children, gliomas were localized mainly in the cerebellum, III and IV ventricles, in adults, mainly in the frontal and parietal lobes. In the subventricular layer of the third ventricle, small nodules corresponding to microastrocytomas and large nodules such as fibrillar astrocytomas were found. The cells formed as a result of proliferation migrated to the periphery of the nodule, which is typical for the patterns of their invasion in tissue culture. This indicates that they belong to spongioblasts differentiating spongioblasts into astrocytes in the center of the nodule. We noted the phenomena of a decrease in the neuroglial component in the area of malignancy and degenerative changes in neurons at the border with the tumor. (Figure 1, 2).
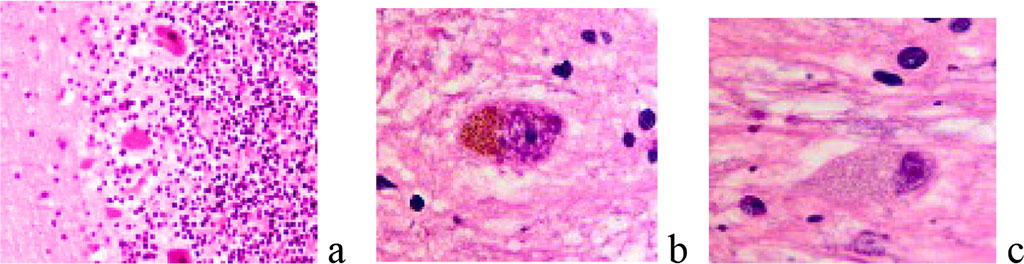
Figure 1 a, b, c - neurons and neuroglia of the brain. A) the norm b, c) degenerating neurons. Stained with hematoxylin and eosin. Increase:а) х100; b-с) х400.
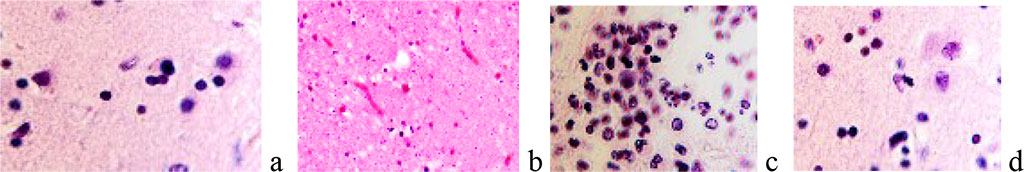
Figure 2. a) neutrophilic macrophage surrounded by neuroglia; b) absence of neurons in the area at the border of the tumor; c, d) apoptosis in the area of malignancy and in the tissue surrounding the tumor. Stained with hematoxylin and eosin. Increase: а) х400; b х100; c, d) х200.
It has been established that the localization of Ki67-positive cells prevails in the structure of the tumor in comparison with its environment (Figure 3).
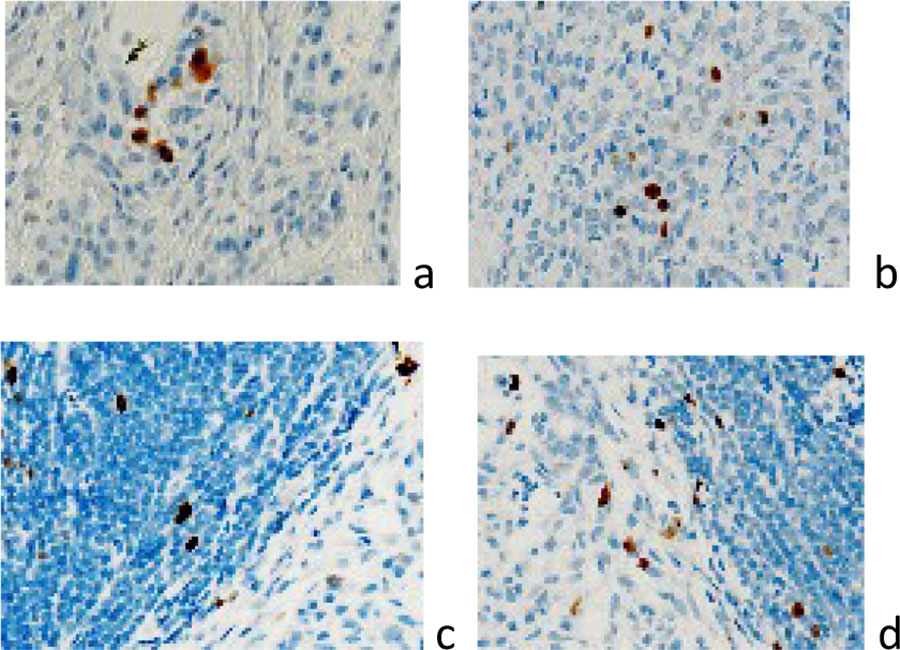
Figure 3 a, b) localization of Ki67 positive cells in the brain tumor structure; c, d) localization of Ki67-positive cells in the tumor structure and at the border with the tumor. Immunohistochemistry for the detection of Ki67-positive cells. Increase: а, b) х400; c, d) х100.
The invasion of tumor cells outside the malignancy zone may be associated with an increase in the Virchow-Robin spaces around the vessels at the borders with the tumor (Figure 4)
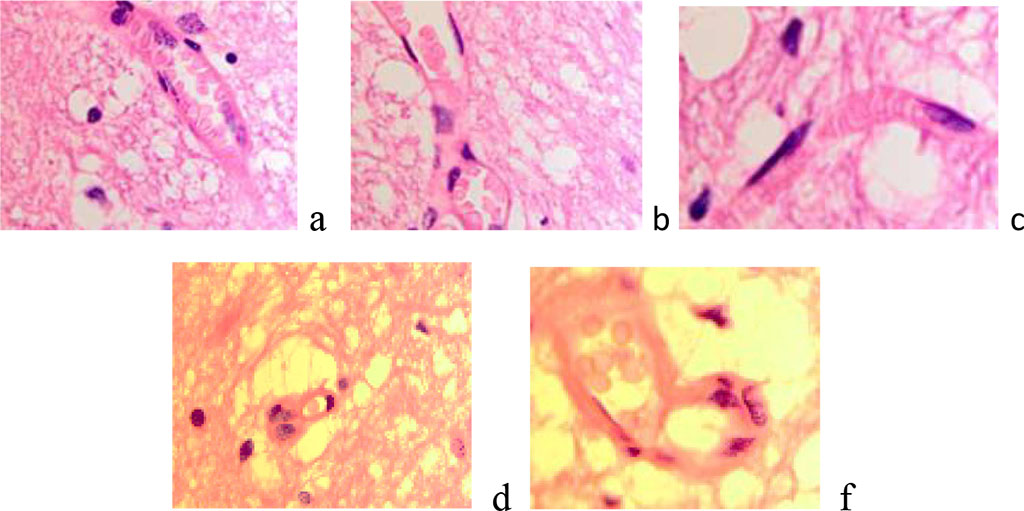
Figure 4 a, b) The blood supply to the brain is normal. C-f) Enlargement of Virchow-Robin spaces in the area at the border with the tumor. Stained with hematoxylin and eosin. Increase х200.
In 3 cases, multiple gliomas were observed, which, according to I.T. Niculescu (1968), may be the result of dissemination with the help of cerebrospinal fluid or have a multifocal origin [8]. We believe that the multiplicity of detected tumors is a confirming fact of the malignancy process, as a generalized process, and not a local one. It is generally accepted that the lack of activity of P53, a proapoptotic factor, leads to malignancy of any tissue. According to our data, as in other tissues, apoptosis in the brain precedes carcinogenesis. In the studied samples, cells of the tumor environment exhibit high activity of the p53 gene (Figure 5).
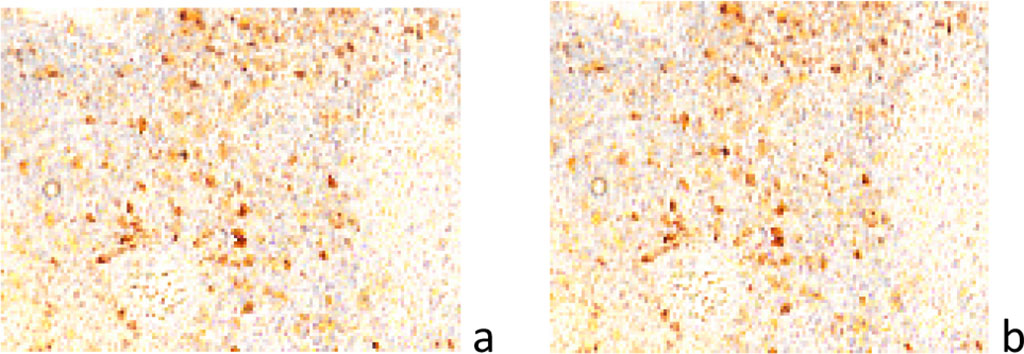
Figure 5 a, b) localization of p53-positive cells in the structure of the tumor and at the border with the tumor. Immunohistochemistry for the detection of p53-positive cells. Increase: а, b) х100.
We associate this fact with the onset of carcinogenesis. It is the preservation of the cytolemma during apoptosis that creates the background of an imaginary well-being of the brain tissue with the absence of inflammatory manifestations and, as a result, inhibition of the recruitment of immunocytes to the start zone of malignancy. After apoptosis of nervous tissue cells, the migrants that settled in the damaged areas have a high regenerative potential and have inducible repressive genes, which is especially important for the p53 genes that ensure programmed cell death in case of damage and genomic disorders.
An important component of the proliferative activity of glioblasts and the lack of their differentiation into specialized cells is the p63 index (Figure 6), as well as their mutual induction with other cells surrounding the malignant zone, which contributes to the invasive spread of the tumor and resistance to therapy.
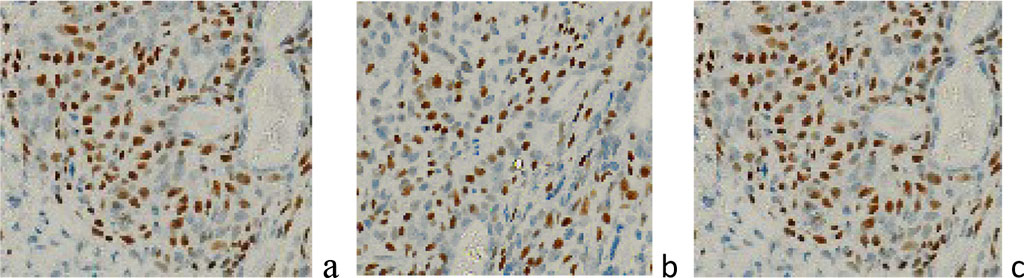
Figure 6 a, b) localization of p63-positive cells in the tumor structure; c) localization of p63-positive cells in the tumor structure and at the border with the tumor of a 45-year-old patient in the area of the right frontal lobe of the brain. Immunohistochemistry for the detection of p63-positive cells. Increase: х400;
Glioblastoma cells, being their own cells of the brain tissue, determine the tolerance of innate cellular immunity to them. In recurrent tumors, an increased content of CD163-positive cells is noted, however, tumor cells suppress the adaptive immune response, which requires a revision of existing concepts of malignancy and additions to new strategies aimed at inducing an antitumor response of the immune system. In the cell ensembles of the tumor and its microenvironment, we identified macrophages/microglia, CD4+ T-lymphocytes, and neutrophils. an increase in the number of macrophages with a relapse of the disease, as well as with relapses after radiation therapy. Recently, data have been obtained on the development of tumors of neural origin in other organs, the nature of which has been proven by immunohistochemical methods. In our opinion, these data only confirm the generalization of the process of impaired development and specialization of cells of various differons in any direction. Since the basis of the tumor contains lymphocytic precursors, the fact of not metastasis, but the colonization of the brain tissue by tumor cells, both with the help of cerebro-spinal fluid and Virchow-Robin spaces, is easily explained. This is confirmed by the fact that systemic carcinogenesis is similar to neurofibromatosis and develops with the simultaneous growth of multiple tumors. The microenvironment produces signals that determine the behavior of stem cells. For example, neural stem cells are present in different parts of the brain, have pluripotency, but only cells localized in certain areas realize this possibility. [9, 10]. At the same time, fibroblast growth factor (FGF-2) can activate latent neural stem cells from different parts of the adult brain. In this regard, the direction of differentiation of stem cell descendants can be determined by the signals that are created in each specific niche. Mature cells and cambial cells disappear in the tumor tissue, malignant tissue is represented by a large number of proliferating polymorphic cells. The activity of p63 confirms the attempt to maintain homeostasis by the nervous tissue of the human brain and the initial differentiation of stem precursor cells. We have noted that the physiological significance of p63 is realized through tumor suppression, excessive expression of this gene is observed in brain tumor tissue and manifests itself in differentiating cells. Gene amplification of the 3q27 locus (where the p63 gene is present) can cause p63 to accumulate in the cancer. DNp63 plays an important role in maintaining stem cells and their ability to differentiate, as noted in our studies. In addition, DNp63 inhibits neuronal aging and apoptosis. It is known that the inhibitory functions of p63 are also associated with early tumor growth in cutaneous squamous cell carcinoma. According to Kadam S.D. et al., (2015), in 80% of head and neck squamous cell carcinoma, the p63 gene is enhanced [11], which corresponds to our data on malignancy of brain structures. The absence of CD4/8 positive effector cells found by us in the malignancy zone can reduce the activity of macrophages in the antitumor immune response, as well as counteract programmed cell death, affecting the outcome and prediction of tumor recurrence. Wang L., Babikir H., Müller S., Yagnik G., Shamardani K., Catalan F., Kohanbash G., Alvarado B., Di Lullo E., Kriegstein A., Shah S.,Wadhwa H., Chang S.M., Phillips J.J., Aghi M.K., Diaz A.A. (2019) it was noted that glioblastoma tumor cells (GBM) have proneural and mesenchymal subtypes, while glioma stem cells (GSC) of the classical subtype have not been identified [12].
The data obtained refute the dogma of CNS immunoprivileging and include the study of the role of immunocytes in the malignancy of brain tissue in postnatal ontogenesis for the development of active immunotherapy using vaccines and cell technologies, immune suppression of carcinogenesis and optimization in the interaction of signaling molecules among the most topical issues. The results can serve as an addition to the fundamental platform in the development of new strategies for antitumor therapy aimed at controlled migration of immunocytes to the malignancy zone and its surroundings, targeted drug delivery for controlled induction of the activity of effector immunocytes in the malignancy zone against the background of impaired signaling interactions. At the present stage, the question of the possibility of differentiation of precursors for neuroglia is controversial, since many authors suggest that the detected astrocytes are residual elements of the malignant nervous tissue of the brain. We consider the multiplicity of detected tumors as a confirming fact of malignancy as a generalized process. Understanding intercellular interactions in the structure of a tumor and its environment and multidimensional connections between a tumor and cells in its environment may open up new avenues for therapy. Like other authors, we noted cellular polymorphism of glioblastomas, but, unlike other data, in our study, 2-nuclear cells were identified, and the absence of multinuclear ones. There was also a decrease in the spectrum of macrophages and the number of cells expressing CD68. The obtained data and analysis of the available literature indicate that malignancy of the nervous tissue of the brain may be due to various intercellular interactions and key signaling pathways involved in immune regulation, tumor proliferation, response to treatment and cellular functions in glioblastoma, which can be reproduced from the outside. Therefore, the strategy of tumor treatment requires not only double inhibition of the mechanisms of pGSC- and mGSC- proliferation and cell survival, but also directed induction on the activation of target immunocytes/phagocytes, which will provide a more complete treatment than combinations that target only cancer cell phenotypes. Aging of non-tumor brain cells contributes to malignancy, which requires premedication before irradiation, to which the astrocyte population is especially susceptible. In addition, stimulation of immuno-phagocytic targets can reduce the aggressiveness of recurrent glioblastomas. This method of treatment has higher prospects in comparison with the standard treatment of surgical resection of the tumor, which induces microglial/macrophage infiltration, angiogenesis, and also as an iatrogenic factor of mediated self-renewal of glioblastoma tumor cells, contributing to therapeutic resistance and tumor recurrence.