- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
We comparatively studied the results of treatment of 86 patients with surgical infectious complications after cavitary intra-abdominal operations for emergency abdominal diseases. In the main group, treatment was supplemented with polyvalent bacteriophages. We studied integral scales for assessing the severity of patient’s condition of (APACHE II, ACI, ASEPSIS), microbial pattern, enteral morphofunctional coefficient, levels of alkaline phosphatase and intestinal alkaline phosphatase, and indicators of cellular immunity. The results of the study show that complex treatment including polyvalent bacteriophages in postoperative purulent-inflammatory complications in urgent surgery reduces the development of antibiotic-resistant strains, as well as the risk of nosocomial microbial wound associations. Enteral application of polyvalent bacteriophages in patients with acute intestinal failure syndrome normalizes microbial enteral imbalance and the level of pathogens contamination of the small intestine. Supplementing the treatment of postoperative abdominal purulent-inflammatory complications with phage therapy can reduce the number of surgical interventions, as well as the treatment duration and mortality.
Keywords: bacteriophages, polyvalent bacteriophages, phage therapy, surgical site infection, abdominal surgery, surgical infection treatment
INTRODUCTION
Surgical site infection (SSI) is one of the most common types of nosocomial infections. SSI is the third most common nosocomial infection, accounting for 10 to 40% of all nosocomial infections. Approximately 0.5% to 3% of surgical patients develop an infection at or near the surgical incision. Compared with postoperative patients without SSI, patients with SSI have approximately 7–11 days longer hospital stays [1–3].
The current assumption that the majority of SSIs that occur after surgery using standard antiseptic methods are associated with intraoperative contamination remains unproven. A growing number of recent studies on the human genome, gut microbiome, and proteomics suggest that loss of mucosal barrier function, especially in the gastrointestinal tract, can significantly affect antigen transport, ultimately affecting the tight bidirectional interaction between the gut microbiome and the immune system. These cross coupling interactions have a great influence on the formation of the function of the host's immune system and, ultimately, on the outcome of interventions. Evidence suggests that pathogens derived from the gut microbiota can cause postoperative infection while they stealthily travel inside an immune cell and contaminate a remote surgical site (Trojan horse hypothesis) [4-8].
The need for new antibiotic therapies is at a crisis level as the number of antibiotic-resistant, multidrug-resistant and extremely drug-resistant bacterial strains increase. Most of pathogen strains are becoming increasingly resistant to antibiotics, and many strains are becoming resistant to multiple antibiotics and chemotherapeutic agents. One possible response to the crisis of antibiotic resistance and the treatment of bacterial infections could be the use of phage therapy. Lytic phages have been shown to be able to selectively infect and lyse multidrug-resistant bacteria, providing an effective antibacterial response in vitro and in vivo. An advantage of phage therapy is the multiple routes of administration of the phage into the body. Local, oral, and intravenous routes of administration allow for tailoring treatment to the site of infection and can be used in conjunction with antibiotic treatment. The current clinical application of phage therapy is hampered by lengthy product development and regulatory approval procedures [9,10].
The purpose of the work was to study the results of the therapeutic use of polyvalent bacteriophages in infectious complications in urgent abdominal surgery.
The objects of the study were 86 patients with various infectious complications after emergency intra-abdominal surgeries performed in 2019-2022. The inclusion criterion in this study was the presence of deep incisional surgical site infection (DISSI) and organ/space surgical site infection (OSSSI). All complications occurred at the inpatient stage of treatment within 1 to 23 days. The exclusion criteria were cases with anticancer chemotherapy, radiation therapy, immunosuppressive therapy. A total of 86 patients were treated, who were divided into two equal comparable groups - the main and comparison groups. The mean age of the patients in the groups ranged from 19 to 82 years. There were 52 men and 34 women. In the main group, the treatment was supplemented by the use of bacteriophages. In the comparison group, treatment was carried out in traditional way, without neither passive nor active immunization methods. (Table 1)
Table 1. Stratification of patients by the nature and severity of complications in the study groups (n=86; abs)
Complication |
Main group (n=43) | Comparison group (n=43) | ||
| Number | Severity by Clavien-Dindo | Number | Severity by Clavien-Dindo | |
| Suture failure | 8 | IIIB-4; IVA-2; IVB-1; V-1 | 7 | IIIB-3; IVA-3; IVB-2 |
| Intra-abdominal abscess | 6 | IIIB-4; IVA-2 | 7 | IIIB-5; IVA-1; |
| Intra-abdominal abscess + eventration | 6 | IIIB-5; IVA-1, V-1 | 5 | IIIB-4; IVA-1; IVB-1 |
| Eventration in a purulent wound | 4 | IIIB-1; IVA-1; IVB-2 | 3 | IVA-1; IVB-2 |
| Liver abscess | 4 | IIIB-2; IVA-1; IVB-1 | 5 | IIIB-4; IVA-1; I |
| Perforation of acute gastrointestinal ulcer | 4 | IVB-3; V-1 | 4 | IVB-3; V-1 |
| Intestinal fistula | 3 | IVB-3 | 3 | IVB-2; V-1 |
| Phlegmon of the anterior abdominal wall | 3 | IVB-2; V-1 | 3 | IVB-3 |
| Subacute peritonitis | 3 | IVB-3, V-1 | 3 | IVB-3 |
| Phlegmon of the retroperitoneal space | 2 | IVB-2; V-2 | 3 | IVB-3; V-1 |
In the main group, traditional treatment was supplemented with the use of polyvalent bacteriophages (PVBP) - filtrate of phagolysates of bacteria Staphylococcus, Streptococcus, Proteus (P. vulgaris, P. mirabilis), Pseudomonas aeruginosa, and enteropathogenic Escherichia coli, Klebsiella pneumoniae (NPO "MICROGEN", Russia). All patients of the main group received the pages orally or through an enteral tube at a dose of 20 ml 3 times a day. In addition, the treatment was supplemented by local application of PVBP. After treatment of a purulent wound or cavity with an antiseptic, it was cleaned with saline, and then a phage was introduced. The dose of bacteriophages for local application depended on the volume of the purulent cavity; it varied from 20 to 200 ml and was injected into the wound or drainage system twice a day. The dose-dependent efficacy of phage therapy was not evaluated in this study. Antibiotic therapy in both groups was carried out in accordance with approved standards and recommendations.
The microbial patterns from the focus of infection of all patients, as well as the enteral luminal pathobiome (of 24 patients of the main group and 23 of the comparison group with nasogastrointestinal drainage) were studied using a VITEK 2 Compact 30 4700733 analyzer (France). Also, we rated colony-forming units (CFU) in 1 ml of wound discharge and small intestine chyme. Antibiotic susceptibility was determined by the EUCAST disk diffusion method (version 2021-01). A total of 237 cultures were taken and 1551 isolates were detected.
Enteral morphofunctional coefficient (EMFC) was dynamically calculated to study enteral insufficiency. Also, we used the method of enzyme immunoassay with the Cobas e411 (Switzerland) to determine total alkaline phosphatase (ALP), its intestinal isoform - intestinal alkaline phosphatase (IAP), as well as their ratio of ALP/ IAP (%) in blood serum and intestinal content.
The phagocytic activity of blood was determined by the phagocytosis of latex by neutrophils. The following indicators were studied: phagocytic number (PN), phagocytosis rate (PR) and the number of active phagocytes (NAP).
The severity of the patients' condition was assessed using the APACHE II (Acute Physiology and Chronic Health Evaluation) scale, the Abdominal Cavity Index (ACI) by V.S. Savelyev, and the ASEPSIS scale. The above indicators were studied on the days 1, 3, 5 and 7 of treatment.
Statistical relationships between indicators were assessed using the correlation module "Basic Statistics and Tables STATISTICA 10.0". We used the adaptive randomization method. To determine the significance of p differences between groups, we used Student's t test and one-way analysis of variance with Fisher's F test. The reliability of data differences in the groups was assessed according to the paired comparison of the Mann-Whitney U-test. Differences were considered statistically significant at p≤0.05.
When studying the wound microbial pattern at the start of treatment, the picture in the main and comparison groups did not differ and was represented by a diverse Gram + and Gram - microflora. In total, 11 pathogens were isolated, which were presented as a monoinfection in 78.4% of cases. In 13.2%, a mix-infection was detected, and in 8.4%, there was no flora growth.
On the day 7 of treatment, the number of mixed infections was statistically higher in the comparison group - 23.4%, in contrast to the main group, where microbial associations were detected only in 7.8% of crops (p≤0.05). Associations in the comparison group were represented by the following combinations: Staphylococcus aureus + Escherichia coli - 32.3%, Staphylococcus aureus + Klebsiella spp. - 13.3%, Staphylococcus aureus + Citrobacter - 11.8%, Staphylococcus aureus + Pseudomonas aeruginosa - 5.4%. In the main group, mixed infections most often occurred as an association of Staphylococcus aureus + Acinetobacter - 36.7%, Staphylococcus aureus + Citrobacter - 8.3%.
At the start of treatment, antibiotic resistance in both groups was comparable and amounted to 3.3% in the main group and 3.6% in the comparison group. On the day 7 of treatment in the comparison group, a statistically greater number of antibiotic-resistant isolates was detected - 12.8%. Moreover, among these strains, MRSA was found in 13.2%, VRSA in 5.3%, and in 4.9% of crops, the microbiota was completely resistant to the studied antibiotics. At the same time, the number of resistant strains in the main group remained almost unchanged at 3.4% (p≤0.05). No cases of MRSA and VRSA were registered in the main group.
When studying microbial contamination at the start of treatment, high rates were noted in both groups, which ranged from 4.5±0.3X1012 CFU to 2.3±0.2X108 CFU. On the day 7 of treatment, there were no statistically significant differences in the degree of microbial contamination in the main and comparison groups; the values were 4.2±0.2X104 CFU and 3.8±0.2X104 CFU, respectively (p≥0.05).
When studying the enteral luminal pathobiome, on the 1st day of treatment, Gram-pathogens (Klebsiella spp., Escherichia spp., Proteus spp., Clostridium spp.) predominated. We noted high contamination by Staphylococcus spp. - a kind of pathogens noncharacteristic for the small intestine.
On the day 5 of treatment, the enteral pathobiome in the groups had differences. In the comparison group, high contamination by the above pathogens remained, which ranged from 7.5±0.4X107 CFU to 7.9±0.4X108 CFU. In the main group, with the use of bacteriophages, there was a tendency to normalization of the enteric microbiome, which was mainly represented by characteristic symbiotic microorganisms of the Enterococcus spp. – 5.5±0.3X105 CFU (р≤0.05). (Figure 1)
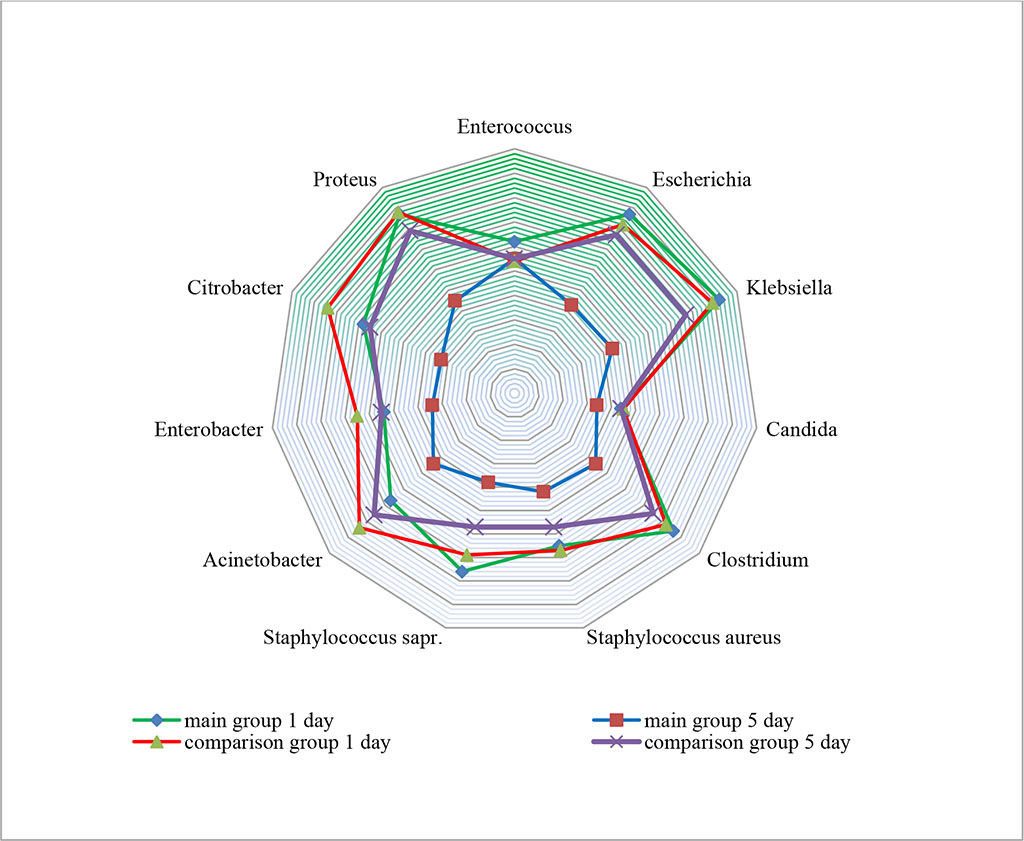
Figure 1. Change in the transluminal enteral pathobiome in study groups (×101-10 CFU in 1 мl)
When comparing the effectiveness of treatment according to the systems for assessing the severity of the patient's condition (APACHE II, ACI), it turned out that statistically significant normalization of indicators occurred earlier in the main group, on average, by the day 5. At the same time, in the comparison group, these changes occurred only by the day 7 of treatment (p≤0.05). Enteral insufficiency stopped earlier in the main group, which was confirmed by changes in EMFC. On the day 5 in the main group it was 4.3±0.3; in the comparison group it did not reach normal values even on the day 7 – it was 7.8±1.1 (p≤0.05). ALP/ IAP values were high in both study groups. In the comparison group, an increase in its level was noted on the day 3 of treatment, which was probably associated with intoxication. At the same time, this ratio in the main group came to normal values (p≤0.05) on the 3rd day Indicators of phagocytic activity of blood increased statistically significantly in the main group by the 3rd day of phage therapy, which was accompanied by an increase in PR and NAP. (Table 2)
Таble 2. Results of treatment in study groups (М±m)
Index |
Study groups (n=86) | |||||||
| Main group (n=43) | Comparison group (n=43) | |||||||
| 1 day | 3 day | 5 day | 7 day | 1 day | 3 day | 5 day | 7 day | |
| Scale APACHE II | 20,3±0,3 | 17,3±0,4 | 10,5±0,4* | 10,7±0,3* | 21,3±0,2 | 18,3±0,3 | 16,5±0,4 | 11,7±0,3* |
| Abdominal Cavity Index | 18,8±0,4 | 16,8±0,4 | 12,6±0,5* | 11,7±0,3* | 19,1±0,5 | 17,9±0,4 | 16,6±0,3 | 12,7±0,3* |
| Enteral morphofunctional coefficient | 26,4±2,6 | 14,3±1,7 | 4,3±0,3* | 3,3±0,2* | 25,4±1,7 | 20,2±2,1 | 16,5±2,2 | 7,8±1,1* |
| Scale ASEPSIS | 32,6±1,6ꜜ | 52,2±2,7 | ||||||
| Alkaline phosphatase/intestinal alkaline phosphatase, blood (%) | 0,71±0,14 | 0,62±0,06 | 0,42±0,08* | 0,43±0,11* | 0,82±0,07 | 1,34±0,09* | 0,92±0,11 | 0,88±0,13 |
| Alkaline phosphatase/intestinal alkaline phosphatase, chyme (%) | 6,21±0,16 | 3,17±0,11* | 3,22±0,08* | 3,11±0,09* | 6,11±0,14 | 8,65±0,14 | 7,99±0,15 | 6,55±0,21 |
| Phagocytosis rate (N 65-95%) | 62,4±3,2 | 71,2±4,4* | 89,2±5,4* | 94,3±6,8* | 61,8±4,4 | 63,2±3,7 | 64,5±6,6 | 66,3±7,1 |
| Phagocytic number (N 4,0-10,0) | 3,5±0,4 | 4,3±0,3 | 8,8±0,7* | 9,7±1,2* | 3,3±0,3 | 3,4±0,3 | 3,7±0,3 | 3,9±0,1 |
| Number of active phagocytes (N 2,5-2,9 thousand in 1 mm3.) | 1,8±0,3 | 2,7±0,2* | 2,7±0,3* | 3,1±0,2* | 1,5±0,5 | 1,9±0,4 | 2,1±0,3 | 2,4±0,1 |
| Average number of surgeries per patient | 2,8 |
4,1 |
||||||
| Duration of hospitalization | 14,7 | 22,3 | ||||||
| Mortality | 3 (6,9%) | 7 (16,2%) | ||||||
Note: *-values at p≤0.05
In the comparison group, patients required more surgical interventions. These were mainly surgical debridements, necrectomy and secondary sutures. We noted a decrease in the duration of inpatient treatment in the main group by 7.6 days. Mortality in the comparison group was more than 2 times higher than in the main group.
Our current experience with the use of polyvalent bacteriophages has shown that phage therapy can be used as an adjunct to the treatment of postoperative purulent-inflammatory complications in emergency surgery.
Of course, a number of questions remain. First of all, how soon resistance to phages develops. According to some authors, it can form after 2-3 weeks of therapy, which was confirmed by the presence of IgG antibodies. There are questions about the use of phage therapy in severe sepsis and septic shock. Despite the fact that no significant immunological complications of phage therapy have been reported in studies, the question arises of possible immunogenicity due to the massive lysis of bacterial cells. Although the patient's condition itself, accompanied by a systemic inflammatory response syndrome, and bacteremia which requires systemic antibiotic therapy, is life-threatening. The complex interactions of the immune system functioning to its limit, bacteria, and phages are difficult to both model and predict [11,12].
After the microbiological studies, there is an urgent need for the development and clinical application of new phages, in particular against such pathogens as Citobacter and Acinetobacter.
Complex treatment including polyvalent bacteriophages in postoperative purulent-inflammatory complications in urgent surgery reduces the development of antibiotic-resistant strains, as well as the risk of nosocomial microbial wound associations.
Enteral application of polyvalent bacteriophages in patients with acute intestinal failure syndrome normalizes enteral microbial imbalance and the level of contamination of the small intestine by pathogens.
Supplementing the treatment of postoperative abdominal purulent-inflammatory complications with phage therapy can reduce the number of surgical interventions, as well as the duration of treatment and mortality.