- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 4: e1. DOI 10.35630/2199-885X/2022/12/4.8
Aim of our investigation is to evaluate the use of the device for modeling of intra-arterial circulation and substantiate the possibility of its use in experimental cardiology.
Materials and methods. The principle part of the model is the cone-shaped narrowed glass cylinder 365 mm long with an inlet diameter of 20 mm, an outlet diameter of 16,5 mm. Two-way fitting with a rubber valve is screwed from the input side. Flexible silicone tubes are attached to both sides of the cylinder, with the other end attached to an electric water pump. The water pump is connected with 12 Volt battery. In one of the outer corners, we insert a needle for introducing dyes (clerical ink) and a silk thread through the fitting valve into the glass tube. We filled the closed circuit with an aqueous solution of glycerin, the viscosity of which was similar to the blood. The water pump provides continuous or intermittent circulation of the glycerin solution in a closed system. We observed the thread movements and turbulent flow colored by the ink during the spread of the pulse waves.
Results. We simulated the pulse waves occurring in the regular heart rhythm, as well as arrhythmias. When simulating the extrasystoles, we observed a turbulent fluid flow during the spread of the 1st post-extrasystolic wave behind the installed diaphragm. With the spreading of the 1st post-extrasystolic wave, the pressure level inside the tube is increased in comparison with the regular rhythm.
Conclusion. Device for modeling of intra-arterial circulation is simple and reliable in its construction. It imitates the intra-arterial processes in different hemodynamic situations, such as regular heart rhythm, arrhythmias, and atherosclerotic plaques. It can be used in the wide spectrum of experimental works in cardiology, vascular surgery, physiology, pathophysiology, biophysics.
Keywords: modeling of intra-arterial circulation, experimental cardiology, device for modeling, simulating of circulation.
Nowadays the medical science is difficult to exist without experiments [1-3]. Experimental models have great importance in many cases to confirm the theories and hypotheses. When proving their theories, a researcher often encounters some difficulties in clinical practice, in particular in cardiology. The experimental part of the work becomes impossible to do, and many promising theories are difficult to confirm from the point of view of the principles of evidence-based medicine. Most of the existing experimental models are made for the heart, cardiomyocites, use the computer modeling, but not demonstrate the arterial vessels and do not use physical modeling principles [4-9].
In our clinical practice, we observed changes in the hemodynamic parameters measured by methods of Doppler ultrasound imaging and digital sphygmography in different arrhythmias (for example, extrasystoles, atrial fibrillation) [10-13]. To confirm the revealed pattern, we have created an original experimental device for modeling of intra-arterial circulation. With its help it would be possible to reproduce and study the hemodynamic processes inside the arteries both in the regular heart rhythm and in various arrhythmias. We realize that in physical modeling, it is not possible to make the absolute identical model as the human arterial vessel, but our construction is very close in functioning and demonstrating the intra-arterial processes.
Aim of our investigation is to evaluate the use of the device for modeling of intra-arterial circulation and substantiate the possibility of its use in experimental cardiology.
The main things in the process of creating the device for modeling of intra-arterial circulation were simulating an arterial vessel, selection of liquid very close to blood viscosity, and ensuring the functioning of the blood flow inside the system, corresponding to the pulse waves in human cardiovascular system, both in regular heart rhythm and in different arrhythmias.
Figure 1 demonstrates the device for modeling of intra-arterial circulation (photo and graphic scheme).

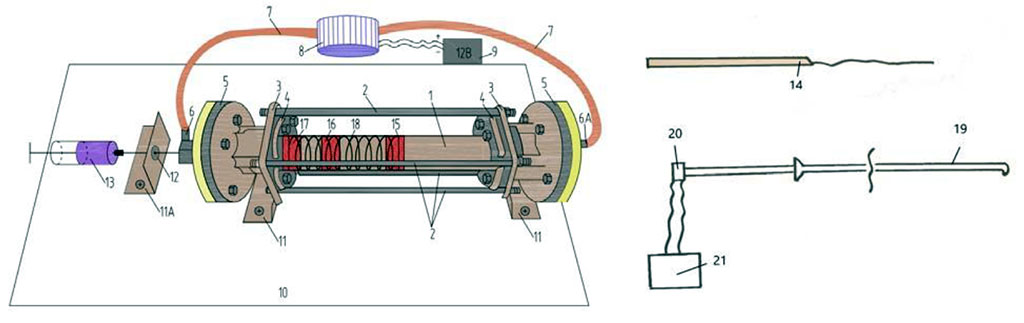
Figure 1. Device for modeling of intra-arterial circulation (descriptions of numbers see in text).
The main part of the model is the cone-shaped narrowed glass cylinder (1) 365 mm long with an inlet diameter of 20 mm, an outlet diameter of 16,5 mm and wall thickness of 2,5 mm. Four steel rods (2) 365 mm long, 1 cm in diameter, 1 cm thick, are fixed parallel to the cylinder. We use two rounded couplings with holes for a glass cylinder and recesses for steel rods (4) for additional fixation of steel rods. The thickness of the couplings was 5 mm. We attach two steel cylinders (5) with a diameter of 10 cm, with a thread in the middle, to the fixing couplings from the outer sides through rubber gaskets 1 mm thick by nut connections. Two-way fitting (6) with a rubber valve is screwed from the input side. Flexible silicone tubes (7) are attached to both sides of the cylinder, with the other end - to an electric water pump (8). The water pump is connected with 12 Volt battery (9). The device is fixed to a horizontal board (10) using three steel angles (11). In one of the outer corners at the level of the fitting there is a hole for the needle (12) for introducing dyes (clerical ink) (13) and a conductor with silk thread (14) through the fitting valve into the glass tube. (15), (16) and (17) – plastic diaphragms invented inside the tube rotameter, imitating the atherosclerotic plaques with internal stenosis of 50%, 70% and 90%. (18) – imitation of spirally oriented muscular fibers in arterial vessel.
We fill the closed circuit with an aqueous solution of glycerin, the viscosity of which is similar to the blood. The water pump provides continuous or intermittent circulation of the glycerin solution in a closed system. We insert a silk thread flexible length fixed at one end to a metal needle, before, inside or behind the diaphragm. We observe the thread movements during the spread of the pulse waves. Simultaneously we put the ink through the fitting valve. With both these indicators (silk thread and ink), we are able to observe the hemodynamic processes inside the glass tube. We also insert a plastic flexible catheter (19) connected to a pressure sensor (20) into the tube, the data of which are registered by an oscilloscope (21). During the experiment, we perform the visual observation of the liquid flow and measure the pressure at various time and different parts in the system.
The device for modeling of intra-arterial circulation has the transparent rotameter glass tube with different diameters at the inlet and outlet parts. The variable tube diameter imitates the native arterial vessels, where the distal part is narrower than the proximal one. The transparent tube is very important for the experimental part, because it makes possible to visualize the hemodynamic processes inside the tube. A closed circuit allows continuous circulation of fluid within the system. To do this, we attached silicone tubes to the inlet and outlet ends of the tube. The free ends of the silicone tubes are attached to an electric pump powered by a 12 Volt battery. The pump is able to work in a pulsed mode, simulating the regular heart rhythm, different types of arrhythmias (extrasystoles, atrial fibrillation etc.) and to create pulse waves.
As a liquid filling the system, we use an aqueous solution of glycerin with viscosity corresponded to the human blood. Using glycerin makes possible to perform the experiment demonstrative (as it is transparent), but close to the conditions of the arterial system functioning.
Main characteristics of the device are presented in Table 1.
Table 1. Main characteristics of the device for modeling of intra-arterial circulation
| No |
Type of diaphragm | Dtube., mm | d., mm | Stube, mm2 | S., mm2 | (S./Stube) ×100 % | Stenosis, % | V, m/sec |
| 1 | 1 | 17,96 | 6 | 253,21 | 28,26 | 11,16 | 88,84 | 8,96 |
| 2 | 18,01 | 10 | 254,62 | 78,50 | 30,83 | 69,17 | 3,24 | |
| 3 | 18,09 | 12,5 | 256,89 | 122,66 | 47,75 | 52,25 | 2,09 | |
| 4 | 2 | 18,19 | 10 | 259,74 | 78,5 | 30,22 | 69,78 | 3,31 |
Notes. Dtube - inner diameter of the rotameter tube in the area of installed diaphragm; d - aperture diameter of the diaphragm; Stube - cross-sectional area of the tube; S - cross-sectional area of the diaphragm aperture; V = Dtube2/d2 – flow rate for the fluid in the aperture of the diaphragm at a flow rate of 1 m/sec; 1 type of diaphragm - with a symmetrical inner hole; diaphragm type 2 - with an asymmetric inner hole.
We simulate the pulse waves in the regular heart rhythm, as well as in extrasystoles and atrial fibrillation. When simulating extrasystolic arrhythmia, we observe the turbulent fluid flow during the spread of the 1st post-extrasystolic wave behind the installed diaphragm (see Figure 2).
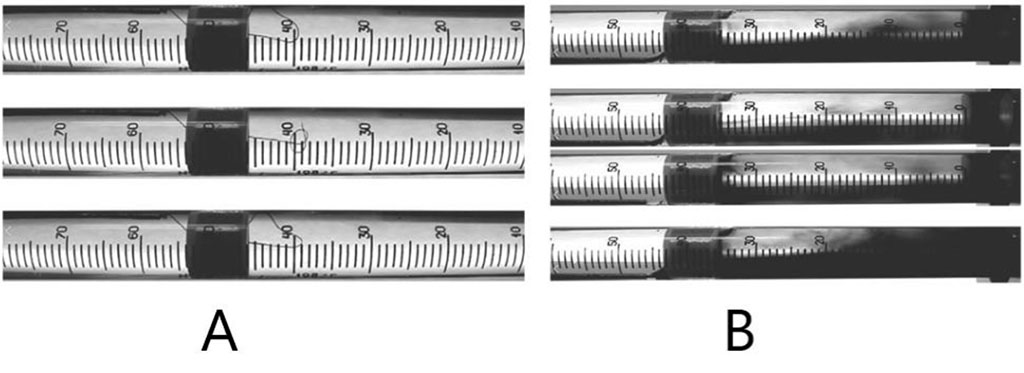
Figure 2. Turbulent liquid flow behind the diaphragm in the 1st post-extrasystolic wave imitation (A - indicator – silk thread, B - indicator – ink).
Figure 3 demonstrates the data from an oscilloscope from the piezoelectric pressure probe while simulating the extrasystolic arrhythmia. With the spread of the 1st post-extrasystolic wave, the pressure level inside the tube is increased.
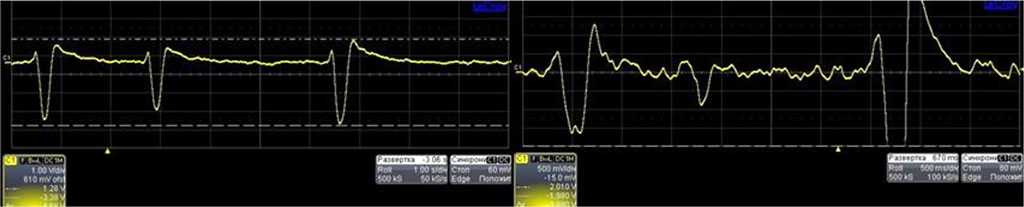
Figure 3. Data from oscilloscope – measured by pressure probe in extrasystolic arrhythmia.
Most of the investigations, in the field of cardiology, including experimental, are focused on the heart [4-9]. However, nowadays there is a rising number of works studying the pathophysiological processes in arterial vessels. It is very helpful when the clinical patterns are confirmed or visualized by the experiments. Experiments on the humans are very limited due to the ethical reasons. Therefore, experimental devices in vitro are very useful. In our work, we created the original device for modeling of intra-arterial circulation. We did the simple construction that every scientist can repeat and reproduce in his laboratory. The device is performed to imitate the blood circulation in the arterial part of cardiovascular system and includes transparent tube, with different inlet and outlet diameter, solution with maximum close viscosity to the human blood, diaphragms imitating the atherosclerotic plaques. Due to the possibilities of the water pump modes, we can imitate the regular heart rhythm, different types of arrhythmias (for example, extrasystoles, atrial fibrillation and others), study the characteristics of the intra-arterial pressure changing, and visualize the intra-arterial hemodynamic processes. It is very simple to change the internal parts in the device up to the necessary task.
Device for modeling of intra-arterial circulation is simple and reliable in its construction. It imitates the intra-arterial processes in different hemodynamic situations, such as regular heart rhythm, arrhythmias, and atherosclerotic plaques. It can be used in the wide spectrum of experimental works in cardiology, vascular surgery, physiology, pathophysiology, biophysics.