- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 4: e1. DOI 10.35630/2199-885X/2022/12/4.10
Objectives. To compare two methods of COVID-19 treatment based on hematology test results and oral cavity manifestations.
Materials and methods. The first part of the study included patients in the period from May to July 2020 with coronavirus infection; another part of the study was conducted in the period from August to October 2020. Two groups received different treatment: 1) with hydroxychloroquine and 2) with without hydroxychloroquine.
Results. C-reactive protein was elevated in 173 (89%) in the hydroxychloroquine group and in 30 (81%) patients in group without hydroxychloroquine (p<.01). As well, INR, Quick prothrombin, D-dimer and neutrophils were statistically different. Hydroxychloroquine treatment showed a decrease in symptoms within 7-10 days while Vitamin C protocol demonstrated health improvements in 4-5 days. Patients from hydroxychloroquine group demonstrated pigmentation in the attached gingiva on both maxilla and mandibula (n=80; 41.4%) and in hard palate (n=3; 1.5%) Lentigo was revealed in 23 (10.8%) patients in both lips. None of the patients from the second group had any of manifestations.
Conclusion. Oral changes are not primary in the oral cavity in COVID-19.
Keywords: COVID 19, SARS-CoV-2, oral mucosa, hydroxychloroquine, direct anticoagulants.
In December, 2019, Wuhan, Hubei province, China, became the outbreak center of the coronavirus infection (COVID-19) caused by the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) [1] and its alarmingly rapid transmission [2] have led to a global health emergency in January 30, 2020 [3].
Coronaviruses are single-stranded RNA viruses with a diameter of 80–120 nm. α-coronavirus, β-coronavirus, δ-coronavirus and γ-coronavirus are viruses that are known nowadays [4]. SARS-CoV-2 is a β-coronavirus. SARS-CoV-2 is more relative to SARS-like bats coronaviruses [5]. Epidemiological studies have shown that the spread of the virus is associated with the different factors such as the source of infection, transmission and susceptibility [6,7].
SARS-CoV-2 causes an acute viral infection in humans with an average incubation period of 3 days [8]. The most common symptoms of COVID-19 are fever, cough and fatigue [9,10]. The most common laboratory abnormalities include decreased lymphocytes [11] and increased alanine aminotransferase and asparagine aminotransferase [12,13], increased proinflammatory cytokines such as interleukin (IL)-1β, IL-6, and increased TNF-α, D-dimer, C-reactive protein [14]. Also, levels of fibrin degradation products and prolonged prothrombin time have been associated with a poor prognosis in patients affected by SARS-CoV-2 [15].
There are four possible drug treatments for COVID-19: Western antiviral medicine, Chinese medicine, enzyme immunoassay and the use of virus-specific plasma globulin [16]. As well some authors [17,18] suggested that Vitamin C has a beneficial effect in COVID-19 treatment due to its antiviral properties by supporting lymphocyte activity, increasing interferon-α production, modulating cytokines, reducing inflammation, improving endothelial dysfunction, and restoring mitochondrial function [19-21]. However, one of systematic reviews has demonstrated that there is no evidence to support or refute the use of vitamin C in the treatment of patients with COVID-19 [22].
The aim of this study was to compare two methods of COVID-19 treatment based on blood test results and oral cavity manifestations. In total this study 230 patients with laboratory confirmed diagnosis.
The first part of the study included patients who were placed in infectious disease wards in Moscow, Russia, in the period from May to July 2020 with a primary diagnosis upon admission of ICD-X: J18.9: coronavirus infection, not laboratory-confirmed, community-acquired bilateral polysegmental pneumonia of moderate severity.
This part included 193 patients in the age span from 24 to 83 years (98 men and 95 women, mean age 57.6 ± 6.3 years). Thirty one of these patients had hypertension, eight patients had diabetes mellitus, one patient had hepatitis A and one patient had chronic bronchitis in the medical history.
These patients were prescribed a ward regimen and oxygen therapy. In most cases, drug treatment protocol included hydroxychloroquine 200 mg, 2 tablets twice a day for a day, then 1 tablet twice a day for 7 days; azithromycin 500 mg 1 tablet once a day for 5 days, direct anticoagulants: clexane, fraxiparin, heparin. Other drugs including paracetamol, 3rd and 4th generation cephalosporins, ketorol, infibeta, kaletra, tocilizumab were prescribed depending on the dynamics of the disease. Also, ten patients received dexamethasone 12 mg intravenously twice a day for two to three days. Further this group is called hydroxychloroquine group.
Another part of the study was conducted in Moscow, Russia, in the period from October to December 2020. Upon admission of a patient with U 07.1 (a coronavirus infection caused by the Covid-19) virus was laboratory confirmed. This part included 77 patients in the age span from 26 to 67 years (30 men and 37 women, mean age 45.2 ± 12.1 years). Further this group is called vitamin C group.
The following therapy was provided based on the experience and previous tests with other patients. From the first day of admission:
Laboratory confirmation was performed using the AmpliSens® (Cov-Bat-FL test system, Russia). A radiographic study of the chest region was performed using multislice computed tomography. Computed tomography data were not documented. Further, upon admission, the patients underwent a general blood test with a leukocyte study, blood biochemistry and screening of the hemostasis system.
The examination was carried out 7 days after patients’ admitting to the departments. The assessment included visual inspection and photographic documentation. Written consent for the examination was given by all patients.
One-way ANOVA test was provided in StatPlus 6 software (AnalystSoft, CA, USA) for the mean data obtained in each group. The level of significance was set at p <0,01.
In total this study 230 patients with laboratory confirmed diagnosis. None of the patients took anticoagulants before testing. One of the patients was excluded from the vitamin C group due to taking antibiotics by his own. The data of this patient will be presented in this article as well, but not in the common group comparison. The average lung damage according to MSCT was 29.1 ± 8.6% (from 6.6% to 52%) for the first group and 17.3 ± 4.1% (from 5% to 32%) for the second group.
The comparative data of coagulological studies and hematology are presented in tables 1 and 2 respectively. C-reactive protein was elevated in 173 (89%) on admission in the hydroxychloroquine group and in 30 (81%) patients in the vitamin C group (p<.01). As well, INR, Quick prothrombin, D-dimer and neutrophils were statistically different.
Table 1. Сoagulation testing.
| Indicator | Average values for the hydroxychloroquine group (first group) | Average values for the vitamin C group (second group) | p-value |
| Prothrombin time (sec) | 11.2 ± 1.1 | 11.9 ± 7.2 | 0.3 |
| INR | 1.18 ± 3.2 | 0.98 ± 0.04 | <.01 |
| Quick prothrombin (%) | 81 ± 9.9 | 103 ± 8.7 | <.01 |
| APTT (sec) | 30.7 ± 2.0 | 32.8 ± 2.2 | 0.5 |
| Fibrinogen (g / L) | 4.6 ± 1.1 | 4.5 ± 0.9 | 0.5 |
| D-dimer (ng / ml) | 355.2 ± 101.76 | 156 ± 79.1 | <.01 |
Table 2. Hematology testing
| Indicator | Average values for the hydroxychloroquine group (first group) | Average values for the vitamin C group (second group) | p-value |
| WBC (10*9/L) | 5.3 ± 1.5 | 4.8 ± 1.2 | 0.1 |
| RBC (10*12/L) | 4.63 ± 0.5 | 5.03 ± 0.4 | 0.6 |
| Hemoglobin (g / L) | 140.1 ± 2.1 | 184.1 ± 59.7 | 0.1 |
| Hematocrit (%) | 40.6 ± 2.3 | 45.5 ± 3.7 | 0.4 |
| Average erythrocyte volume (fl) | 80.2 ± 4.3 | 90.2 ± 2.4 | 0.6 |
| Platelets (10 * 9 / L) | 210.2 ± 62.1 | 196.6 ± 35.2 | 0.3 |
| Average platelet volume (fl) | 8.1 ± 1.5 | 10.1 ± 1.2 | 0.1 |
| Neutrophils (10 * 9 / L) | 3.57 ± 1.1 | 2.88 ± 1.2 | 0.1 |
| Neutrophils (%) | 61.2 ± 3 | 51.3 ± 7.35 | <.01 |
| Lymphocytes (10 * 9 / L) | 1.5 ± 0.56 | 1.75 ± 0.4 | 0.04 |
| Lymphocytes (%) | 26.1 ± 6.1 | 35.1 ± 6.82 | 0.02 |
| Monocytes (10 * 9 / L) | 0.5 ± 0.18 | 0.6 ± 0.2 | 0.3 |
| Monocytes (%) | 9.5 ± 2.51 | 12.1 ± 2.5 | 0.02 |
| Eosinophils (10 * 9 / L) | 0.05 ± 0.02 | 0.05 ± 0.06 | 0.9 |
| Eosinophils (%) | 0.5 ± 0.1 | 0.09 ± 1.1 | 0.09 |
| Basophils (10 * 9 / l) | 0.3 ± 0.21 | 0.01 ± 0.01 | 0.4 |
| Basophils (%) | 1.7 ± 3.2 | 0.3 ± 0.2 | 0.04 |
One patient from the vitamin C group who was excluded from the study due to taking antibiotics by his own without prescription had rapidly changes in the indicators such as white blood cells, neutrophils and lymphocytes. Other parameters were without dramatical changes. Blood examinations were held in the first, fourth and seventh days after accident (Figure 1). This patient was afraid of the therapy incorrectness, nevertheless, these indicators were corrected and increased to the normal ones by twelve day. Noticeably, that patients from the second group always had their blood indicators stable.
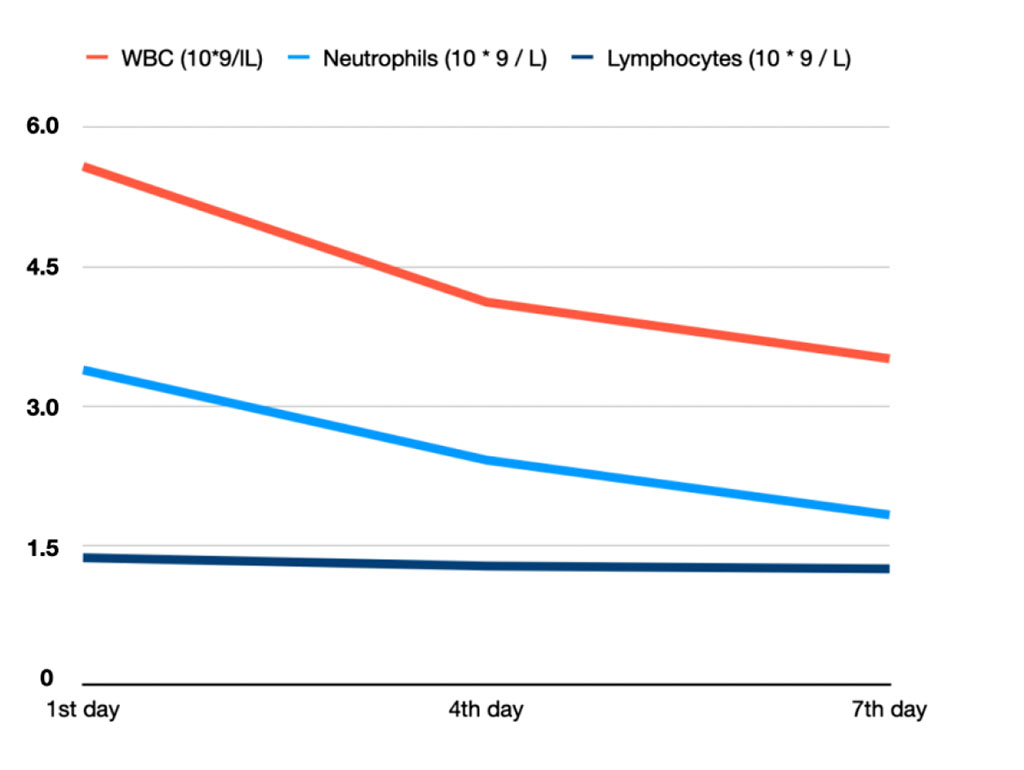
Figure 1. WBC, neutrophil and lymphocyte levels in patient from vitamin C group who took antibiotics.
Examination of the oral cavity of the hydroxychloroquine group in 80 (41.4%) patients revealed pigmentation in the attached gingiva on both maxilla and mandibula (Figure 2) and in 3 (1.5%) patients only in the area of the hard palate. Lentigo was revealed in 23 (10.8%) patients in the upper or lower lip. None of the patient from the vitamin C group had any kind of pigmentation.
Hemorrhagic manifestations occurred in 24 (12.9%) patients, without any pathology in the history in the hydroxychloroquine group. These patients had pronounced cyanosis of the labial mucosa, there was a pronounced vascular pattern on the mucous of the oral surface of the lips. There were also petechiae on the labial and buccal mucosa. As well, none of the patient from the vitamin C group had any kind of hemorrhagic manifestations.
Severe facial xeroderma and angular cheilitis were also noted in 44 (22.7%) patients from the hydroxychloroquine group. These features were not detected in the vitamin C group.
In hydroxychloroquine group 76% of patients during the observation period after 5-7 days, complaints of perverse perception of taste and smell were found, which directed to the diagnosis "R43.1 Parosmia" in 152 patients (78.7%) and "R43.0 Anosmia" in 6 patients (3.1%). “R43.2 Parageusia” was diagnosed in 113 patients (58.5%). In the vitamin C group 37 patients (100%) had "R43.1 Parosmia" diagnosis.
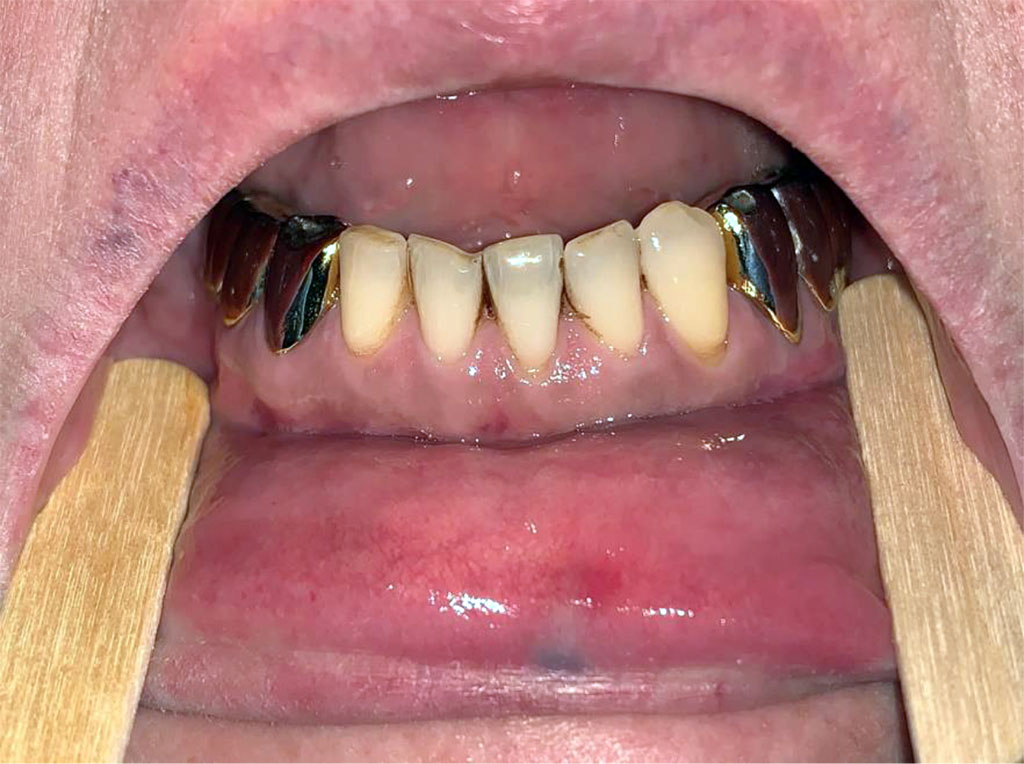
Figure 2. A patient with the lentigo in the lower lip and hyperpigmentation in the mandibular gingiva from the hydroxychloroquine group.
In this study of 270 patients, we analyzed the documentation, which included medical history, radiographic data, blood tests, as well as photographic documentation of the oral cavity. Studied patients’ history were divided in two groups according to treatment protocols.
The first protocol with hydroxychloroquine showed a decrease in symptoms in patients with coronavirus infection within 7-10 days while Vitamin C protocol demonstrated health improvements in 4-5 days. Before starting treatment patients complained of muscular pain, joint pain, “skin pain”, then short cough (during virus “acquaintance” with the pulmonary system), prone position (in patients with labored breathing).
Recent literature review has shown that safety concerns, danger of deprivation of hydroxychloroquine has the questionable efficacy because of the lack of robust clinical evidence to date [23]. In Geleris et al [24] study involving patients with Covid-19 who had been admitted to the hospital, hydroxychloroquine administration was not associated with either a greatly lowered or an increased risk of the composite end point of intubation or death.
Sanders et al. [25] in their pharmacologic treatment review noted that the reported clinical benefits of the combination of hydroxychloroquine and azithromycin for patients with COVID-19 come either from media reports or nonrandomized trials with small numbers of participants (<100 patients). The documented benefit of hydroxychloroquine with or without azithromycin is very limited, especially in severe disease. While these medications, individually or in combination, may prove efficacious, these benefits need to be established with randomized clinical trials prior to widespread adoption of these treatments.
The presented treatment protocol in the second group has following rationale. The expression of the DPP-4/CD26 (human DPP-4/CD26 has been exhibited to connect with the S1 domain of the COVID-19 spike glycoprotein that it is also a salient virulence factor in Covid-19 infection receptor [26]) is reduced significantly in vivo upon the correctness of vitamin D insufficiency [27].
SARS-Cov-2 protease 3CL maintains the same Gln189 site [28] of SARS-Cov 3CLpro, which previously was identified as the binding site for the hydroxyl groups of quercetin and its derivates [29]. Quercetin spontaneously oxidizes to form O-semiquinone and O-quinone/quinone methide (QQ), which can bind protein thiols forming toxic compounds [30]. Vitamin C exerts immunomodulatory activity, enhancing interferon production through STAT3 phosphorylation [31], limiting cytokine-induced organ damage [32], promoting survival in lethal infections [33] and is able to recycle oxidized quercetin [34], enhancing its antiviral effects. SARS-Cov-2 virus infection may initiate a strong inflammatory and dysregulated reaction in the lung with increased levels of IL-6 and a “cytokine-storm” [35] which has been shown to provoke either an asymptomatic, mild, or severe infections. Jang et al [40] identified that vitamin C can reduce the plasma levels of the inflammatory mediators TNF-α and IL-6 via downregulation of hepatic mRNA expression.
Fischer et al. demonstrated that supplementation with vitamins C inhibits the release of IL-6 from contracting human skeletal muscle, thus causing a marked attenuation of IL-6 in the circulation, as well as blunting the increase of IL-1ra and cortisol in response to exercise [53]. That could explain that patients “started feeling awoken and losing muscle-joint pain”.
Relief of symptoms “cytokine-storm” may be supplied with corticosteroids that suppress inflammatory reactions, which were the main means of immunomodulatory therapy during the coronavirus epidemic [36,37], which also made it possible to arrest “cytokine storm” in patients when the corresponding symptoms appeared. Patients showed signs of a cytokine storm (especially patients with increased D-dimer values), namely a complex of symptoms: fatigue, loss of appetite, sharp pain in muscles and joints, nausea, vomiting, diarrhea, rash, rapid breathing, headache, high fever that was not able to be decreased medically, confusion and loss of coordination.
LMWH reduces IL-6 release and activity, which is responsible of the “cytokine storm” as well [38]. LMWH therapy is also associated with better outcomes in severe COVID-19 patients with sepsis-induced coagulopathy and markedly elevated D-dimer levels [39]. As well, it is considered that increasing IL-6 levels are correlated with increasing fibrinogen levels [50].
Zinc is important for the development and maintenance of immune and other cells [41]. Associated information on zinc says that in elderly patients, reduced concentrations of circulating zinc correlated with increased levels of the cytokines IL-6 , IL-8, and TNF-α [42,43].
Oral mucosa hyperpigmentation is common in dark-skinned people due to physiological deposition of melanin. In people with fair skin, this can be an early sign of systemic disease or a side effect of drug therapy [44]. In this study, all patients had fair skin. This pigmentation is probably related to the systemic use of hydroxychloroquine in the first group. Such hyperpigmentation appears as asymptomatic blue-gray spots on the attached gingiva and hard palate [44,45]. It has been previously shown that these areas are the most frequent sites of hyperpigmentation with the use of hydroxychloroquine [46,47].
C-reactive protein is an acute phase inflammatory protein produced by the liver that can be elevated in several conditions such as inflammation, cardiovascular disease, and infection [48]. The recent meta-analysis [49] has shown that despite its value in predicting poor outcome in COVID-19, it should be noted that various factors can affect serum levels, including age, gender, bad habits, weight, lipid levels, blood pressure and liver disorders [48,54].
This study showed that coagulation values, such as INR, Quick prothrombin, D-dimer, were significantly lower in vitamin C group compared to hydroxychloroquine group. Though anticoagulants were given in both protocols, experience and some studies [51,52] dictated that they should be given from the very first day after diagnosis approved. That led to the more precise coagulation control. However, it is still unknown if LMWH alone or in combination with other drugs is useful.
This study showed patients’ tests and oral cavity examinations with COVID 19 (SARS-CoV-2) two protocol treatments. Using vitamin C in combination with other drugs showed faster patients’ convalescence. Oral changes are not primary changes in the oral cavity, despite the fact that the oral cavity is one of entry gate for infection.