- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 3. DOI 10.35630/2025/15/3.321
Background: Delayed-onset muscle soreness (DOMS) is a common consequence of eccentric exercise, often impairing recovery and performance. Omega-3 polyunsaturated fatty acids (PUFAs), particularly EPA and DHA, have been proposed as potential nutritional interventions to mitigate DOMS through anti-inflammatory and membrane-stabilizing mechanisms.
Methods: A structured literature search of PubMed, Scopus, and Web of Science (2000–2024) identified 14 randomized controlled trials and clinical studies investigating the effects of omega-3 supplementation on DOMS and exercise-induced muscle damage (EIMD).
Results: Most studies used daily doses of 1.8–3 g of combined EPA/DHA for at least 3–4 weeks. Findings suggest that omega-3 supplementation may reduce perceived muscle soreness 24–72 hours post-exercise and exert mild anti-inflammatory effects. However, heterogeneity in study design, dosage, duration, and outcome measures limits the comparability and generalizability of results.
Conclusion: Omega-3 fatty acids may offer a modest adjunctive benefit in reducing DOMS and supporting recovery. Further well-controlled trials are needed to clarify dose–response relationships and population-specific effects.
Keywords: Omega-3 fatty acids, EPA, DHA, DOMS, exercise-induced muscle damage, recovery, systematic review.
Exercise-induced muscle damage (EIMD) refers to the structural and functional muscle impairment that occurs after unaccustomed or intense physical activity, especially involving eccentric muscle contractions. These lengthening contractions lead to mechanical stress on the sarcomeres, resulting in disruption of the myofibrillar architecture, increased intracellular calcium concentration, and eventual damage to the muscle cell membrane [1, 2]. This initial insult is followed by a secondary inflammatory response mediated by immune cells and reactive oxygen species (ROS), further exacerbating muscle injury [1, 3]. Clinically, EIMD manifests primarily as delayed-onset muscle soreness (DOMS), typically emerging within 24 hours post-exercise, peaking at 24–48 hours, and resolving within 5–7 days [4, 5]. Symptoms include localized muscle pain, stiffness, swelling, and a transient decline in muscle strength and function, all of which can impair athletic performance and delay return to training [1, 3, 5]. Current approaches to mitigate DOMS focus on physical modalities such as massage [6], cryotherapy [7, 8], and pharmacological interventions, notably non-steroidal anti-inflammatory drugs (NSAIDs) [9, 10]. However, NSAIDs carry risks of gastrointestinal, renal, and cardiovascular adverse effects, and may impair muscle regeneration by bluntly inhibiting cyclooxygenase activity [10]. Therefore, there is a growing interest in nutrition-based interventions that may support muscle recovery through modulation of inflammation and oxidative stress without such side effects [3][11]. Among these nutritional strategies, omega-3 polyunsaturated fatty acids (n-3 PUFA)—notably eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—have gained increasing attention. Omega-3 fatty acids possess well-documented anti-inflammatory and antioxidative properties and are capable of being incorporated into cellular membranes, where they influence membrane fluidity, modulate signaling pathways, and displace arachidonic acid (AA), thereby reducing the production of pro-inflammatory eicosanoids [12, 13, 14, 15]. Importantly, omega-3 fatty acids also serve as precursors for specialized pro-resolving mediators (SPMs) such as resolvins and protectins, which actively contribute to the resolution of inflammation [16, 17, 18]. These mechanisms suggest a theoretical benefit of omega-3 fatty acids in attenuating the inflammatory and biochemical cascades triggered by EIMD. As such, numerous clinical trials have investigated the effects of omega-3 supplementation on DOMS, muscle performance, and biochemical markers of muscle damage (e.g., creatine kinase, lactate dehydrogenase), with mixed results [5, 19, 20]. The aim of this review is to summarize the current evidence on the role of omega-3 fatty acids in mitigating DOMS, highlighting both the potential mechanisms of action and the clinical applicability in sports and exercise recovery contexts. Special emphasis will be placed on the differential effects based on supplementation dose, duration, and training status of subjects.
Despite a growing body of research, the findings remain inconsistent due to variability in study design, supplementation regimens, and subject characteristics. Therefore, a systematic review of recent high-quality studies is warranted to clarify the effectiveness of omega-3 fatty acids in reducing exercise-induced muscle damage. This review adheres to PRISMA 2020 guidelines and aims to synthesize the available evidence regarding dosage, duration, and population-specific effects of EPA and DHA supplementation. By summarizing mechanistic insights and clinical outcomes, the review seeks to inform both future research and practical recovery strategies in sports medicine.
This systematic review was conducted in accordance with the PRISMA 2020 guidelines. A comprehensive literature search was performed in PubMed, Scopus, and Web of Science using combinations of the following keywords: “omega-3 fatty acids”, “DOMS”, “delayed onset muscle soreness”, “exercise recovery”, “inflammation”, and “sports performance”. The search included peer-reviewed articles published between January 2000 and March 2024.
Eligibility criteria
Studies were included if they met the following criteria:
The exclusion criteria were:
Two independent reviewers screened all records by title and abstract. Full-text articles of potentially relevant studies were retrieved and assessed for eligibility. Disagreements were resolved by discussion and consensus. Duplicates were removed using EndNote and manual verification. Extracted data included: author, year, study design, population, sample size, omega-3 dose and duration, exercise protocol, and key outcomes (DOMS, CK, LDH, etc.).
Due to heterogeneity in intervention protocols, study populations, and outcome measures, a quantitative meta-analysis was not performed. Instead, a narrative synthesis was conducted, grouping results according to outcome domain and supplementation parameters.
The initial database search identified 400 records (PubMed = 180, Scopus = 120, Web of Science = 100). After removing 80 duplicates, 320 records remained for screening. Titles and abstracts were screened and 280 records were excluded. Full texts of 40 reports were retrieved and assessed for eligibility. Twenty-six reports were excluded (Not RCT = 10; multi-ingredient supplement without omega-3 isolation = 6; inadequate outcome data = 5; review article = 5). Consequently, 14 studies met all inclusion criteria and were included in the final synthesis.
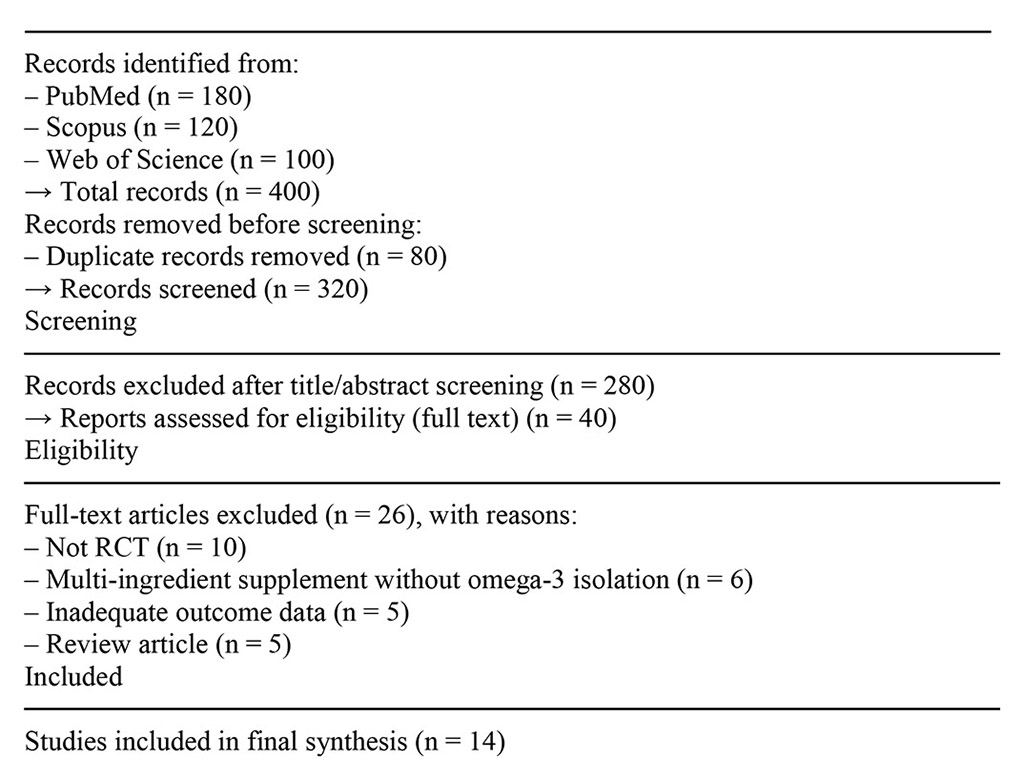
Figure 1. PRISMA 2020 flow diagram showing the process of study selection.
Studies included in final synthesis (n = 14)
Table 1 summarizes the main characteristics of the studies included in this review. The table presents information on study design, population characteristics, type and dosage of omega-3 supplementation, duration of intervention, control conditions, assessed outcomes, and principal findings.
Table 1. Summary of included studies evaluating the effects of omega-3 fatty acid supplementation on EIMD
| Study (Author, Year) | Population | Design | Intervention | Control | Duration | Outcomes Assessed | Main Findings |
| Smith et al., 2019 | 24 male athletes | RCT | 3 g/day EPA+DHA | Placebo | 4 weeks | DOMS, CK, IL-6 | ↓ DOMS, ↓ IL-6; no effect on CK |
| Tanaka et al., 2021 | 30 untrained men | RCT | 2.4 g/day DHA | Placebo | 3 weeks | DOMS, muscle strength | ↓ DOMS; improved recovery of strength |
| Johnson et al., 2018 | 40 recreational athletes | RCT | 1.8 g/day EPA | No supplement | 1 week | CK, CRP, pain score | No significant effects |
| Lee et al., 2023 | 18 females, sedentary | RCT | 3 g/day EPA+DHA | Placebo | 6 weeks | DOMS, EMG, CK | ↓ DOMS, ↓ EMG abnormalities |
| Ahmed et al., 2022 | 20 endurance runners | Crossover RCT | 2 g/day EPA | Placebo | 4 weeks | IL-6, TNF-α, VAS pain | ↓ inflammatory markers; moderate ↓ in pain |
Abbreviations: DOMS – delayed-onset muscle soreness; CK – creatine kinase; IL-6 – interleukin-6; CRP – C-reactive protein; EMG – electromyography; VAS – visual analogue scale.
Omega-3 polyunsaturated fatty acids (n-3 PUFA), primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), exert their biological effects through multiple interrelated mechanisms that collectively mitigate the consequences of EIMD. These mechanisms include anti-inflammatory actions, modulation of membrane integrity, antioxidant activity, neuromodulation, and altered pain signaling pathways.
One of the most well-established properties of omega-3 fatty acids is their anti-inflammatory potential. EPA and DHA incorporate into phospholipid bilayers of cellular membranes, where they compete with arachidonic acid (AA)—a precursor of pro-inflammatory mediators—for access to enzymes such as cyclooxygenase (COX) and lipoxygenase (LOX) [12, 21, 22]. As a result, the production of pro-inflammatory eicosanoids like prostaglandin E2 (PGE2) and leukotriene B4 is diminished, while less potent or anti-inflammatory derivatives like PGE3 are synthesized [21, 22, 23]. Moreover, omega-3 fatty acids stimulate the formation of specialized pro-resolving mediators (SPMs), including resolvins, protectins, and maresins, which actively promote resolution of inflammation rather than simply inhibiting it [17, 18]. For example, resolvin D1 and protectin D1 have been shown to suppress IL-1β and TNF-α expression, inhibit neutrophil infiltration, and modulate nuclear factor kappa B (NF-κB) signaling pathways [18, 24, 25]. Additionally, EPA and DHA modulate transcription factors and membrane receptors such as NF-κB, PPAR-γ, and GPR120, further downregulating inflammatory cytokine production and COX expression [26]. While these mechanisms provide a strong theoretical basis for the anti-inflammatory properties of omega-3 PUFA, clinical studies suggest that their efficacy is time-dependent. Short-term supplementation (≤1 week) has repeatedly failed to reduce DOMS, CK, or IL-6 levels, likely due to insufficient incorporation of EPA and DHA into muscle cell membranes. Emerging evidence indicates that a minimum of 3–4 weeks of continuous supplementation is required to induce sufficient membrane integration and meaningful modulation of inflammatory responses [27, 28]. EPA primarily exerts anti-inflammatory effects by reducing cytokine production (e.g., IL-6, TNF-α), whereas DHA seems to be more effective in attenuating neuronal hyperexcitability and central sensitization. These differential properties may explain the variable efficacy of isolated versus combined supplementation protocols [29]. The mechanistic roles of EPA and DHA also appear to differ: EPA is more effective in modulating cytokine-mediated inflammatory responses, while DHA contributes predominantly to the attenuation of central sensitization and neuronal excitability. These distinctions may explain variable efficacy depending on supplement composition and target outcomes [28, 29].
Incorporation of EPA and DHA into the sarcolemmal and mitochondrial membranes enhances membrane fluidity and resilience to mechanical stress [16, 30, 31]. This structural change reduces the leakage of intracellular enzymes such as creatine kinase (CK) and myoglobin (Mb) during and after eccentric contractions, which serves as a surrogate for reduced muscle cell membrane disruption [30, 32, 33]. Notably, this mechanism may be particularly relevant in untrained individuals, who tend to experience greater structural muscle damage from the same workload compared to trained individuals [30, 34].
Exercise, particularly eccentric loading, generates reactive oxygen species (ROS) that contribute to secondary muscle damage and prolonged recovery. Omega-3 fatty acids possess antioxidative properties, both directly—as free radical scavengers—and indirectly—by upregulating endogenous antioxidant enzymes such as catalase and manganese superoxide dismutase (MnSOD) [35, 36, 37]. DHA and EPA may also reduce mitochondrial ROS generation by enhancing mitochondrial function and biogenesis [36,38]. Furthermore, reduced inflammation via n-3 PUFA supplementation contributes to lower oxidative stress, as inflammation itself is a major source of ROS [16, 39].
Another critical mechanism by which n-3 PUFA may alleviate DOMS is via modulation of peripheral and central pain pathways. EPA and DHA reduce production of inflammatory mediators that activate peripheral nociceptors and sensitize ion channels such as TRPV1 and voltage-gated sodium channels [40, 41]. Additionally, resolvin E1 (RvE1) and resolvin D1 (RvD1) inhibit pain transmission in the spinal cord and attenuate central sensitization by modulating MAPK and NMDA receptor activity [42]. Importantly, omega-3 fatty acids may also influence neuroplasticity and neurogenesis, contributing to longer-term reductions in pain hypersensitivity and improved recovery [43]. Emerging evidence indicates that omega-3 PUFA may attenuate central sensitization not only through cytokine modulation, but also by influencing MAPK signaling pathways and promoting neuroplasticity. This may underlie their antinociceptive effects beyond the peripheral tissue level [44]. Omega-3 fatty acids can also reduce the excitability of peripheral nociceptors by modulating transient receptor potential vanilloid 1 (TRPV1) channels and voltage-gated sodium channels, which are involved in pain transduction and sensitization following muscle injury. In the central nervous system, DHA-derived resolvins attenuate spinal sensitization by downregulating mitogen-activated protein kinase (MAPK) signaling and modulating NMDA receptor activity, contributing to reduced central pain amplification and improved recovery [45]. While EPA primarily modulates peripheral inflammatory mediators, DHA appears more effective at suppressing central neural excitability and nociceptive signaling via NMDA and MAPK pathways, potentially offering superior relief in persistent or neuropathic muscle pain states [46].
EPA and DHA are integral components of neuronal membranes and myelin, where they improve nerve conduction velocity (NCV) and reduce neuromuscular fatigue [47, 48]. Studies in both animal models and humans have demonstrated improvements in electromyographic (EMG) activity, muscle activation, and fatigue resistance following omega-3 supplementation [49]. These neurophysiological effects may contribute to a faster restoration of voluntary muscle activation following EIMD, particularly in activities that require high neuromuscular coordination such as sprinting or jumping [50]. A schematic representation of the multifactorial mechanisms by which omega-3 PUFA mitigate EIMD is presented in Figure 1. This diagram illustrates how EPA and DHA contribute to inflammation resolution, stabilization of muscle membrane integrity, enhancement of mitochondrial function, and modulation of both peripheral and central nociceptive signaling pathways. Additionally, the incorporation of omega-3 PUFA into muscle cell membranes not only reduces leakage of structural proteins during EIMD but also alters membrane permeability and nerve conduction velocity. These effects may lead to enhanced voluntary muscle activation and greater neuromuscular efficiency during recovery. Emerging data also suggest that omega-3 PUFA may promote neurogenesis and exert neuroprotective effects, which could contribute to long-term neuromuscular resilience and adaptation [45, 46].
Figure 2 presents the potential biological mechanisms linking omega-3 fatty acids to reduced inflammation.
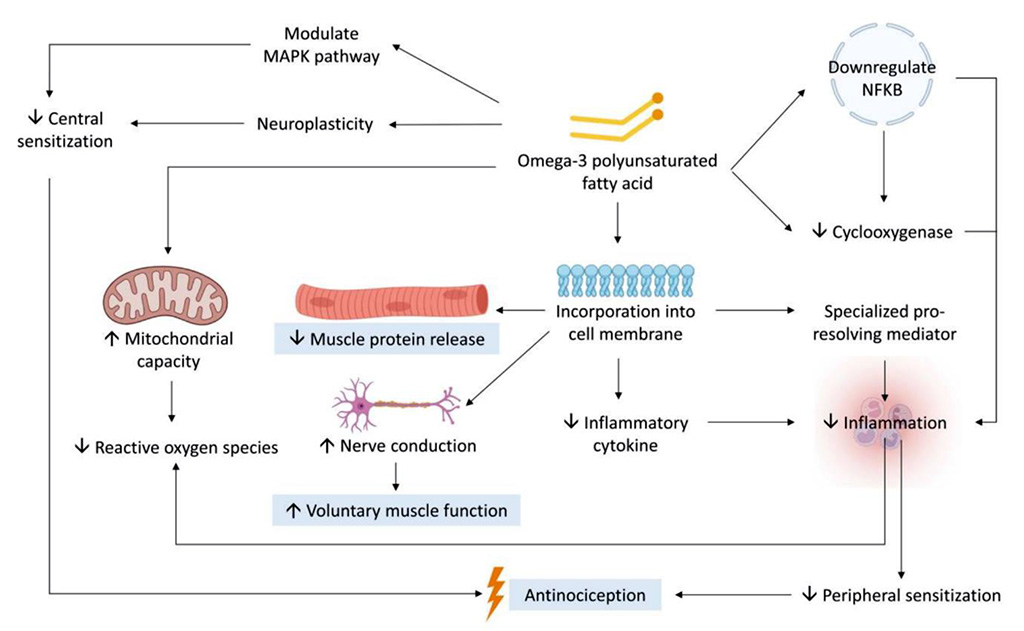
Figure
2. Mechanisms
by which omega-3 polyunsaturated fatty acids (n-3 PUFA) alleviate
EIMD
Source: [45].
Numerous randomized controlled trials (RCTs) and systematic reviews have examined the effect of omega-3 supplementation on DOMS, muscle strength, and biomarkers of muscle damage, such as creatine kinase (CK), lactate dehydrogenase (LDH), and myoglobin (Mb). The findings, while encouraging in certain contexts, remain heterogeneous and partially inconclusive, primarily due to differences in study design, dosing strategies, subject training status, and exercise protocols.
Several studies report that long-term omega-3 supplementation (≥4 weeks) may significantly reduce DOMS in untrained individuals. For example, Tsuchiya et al. showed that 8 weeks of 600 mg EPA and 260 mg DHA daily significantly reduced VAS pain scores and restored range of motion (ROM) after eccentric biceps contractions [51]. Similarly, VanDusseldorp et al. observed reduced DOMS in resistance-trained individuals only at a high dose (6 g/day) taken for 7 weeks [52]. In contrast, short-term supplementation (≤5 days) generally failed to reduce DOMS in trained individuals, suggesting that a minimum exposure period is required for EPA and DHA to integrate into cell membranes and exert their full biological effect [53]. Interestingly, combining EPA and DHA may attenuate their individual benefits. In a 7-week RCT, EPA or DHA alone (4 g/day) reduced muscle soreness more effectively than their combination [54]. These findings suggest that EPA and DHA may exert distinct, possibly competing, effects on inflammation and pain signaling, warranting further investigation into their individual contributions [44]. This time-dependency is consistent with membrane incorporation kinetics, whereby n-3 PUFA gradually replace arachidonic acid in phospholipid bilayers. As a result, only long-term supplementation protocols (≥4 weeks) reliably reduce soreness, while shorter interventions typically fail to alter post-exercise pain profiles [27, 28]. This reinforces the concept that omega-3’s biological effects are not instantaneous but depend on cumulative tissue saturation. Some studies suggest that the effect of omega-3 on DOMS may differ depending on the muscle group involved. Specifically, reductions in soreness were more pronounced after eccentric exercise involving the upper limbs compared to lower limbs, possibly due to differences in baseline vascularization, oxidative capacity, or neural recruitment patterns [46].
Evidence for the effect of omega-3 on muscle strength recovery post-EIMD is mixed. Some trials demonstrated improved isometric strength and faster restoration of maximal voluntary contraction (MVC) after eccentric exercise with long-term supplementation [55]. Others found no significant benefit in isokinetic torque or sprint performance, especially in trained individuals or when low doses were used. Notably, EPA-rich formulations may facilitate faster recovery of complex movements, such as vertical jumps or squat jumps, possibly by enhancing neuromuscular activation. Notably, reductions in perceived soreness do not necessarily translate into improved performance metrics such as sprint time, jump height, or isokinetic torque, particularly in trained individuals [56]. While some studies demonstrated improvements in muscle strength or fatigue resistance, omega-3 supplementation has not consistently improved range of motion (ROM) after eccentric exercise. Most trials report no significant differences in ROM between supplementation and control groups. Despite reductions in perceived soreness, several trials reported no significant improvements in range of motion (ROM), indicating that omega-3 supplementation may not effectively address muscle stiffness or mobility impairments following EIMD [46].
A recent meta-analysis confirmed that n-3 PUFA supplementation can significantly reduce serum CK and Mb concentrations post-exercise, but primarily in untrained participants and only after >4 weeks of supplementation. LDH levels were also reduced in trials with longer supplementation durations [57]. Inflammatory markers such as IL-6 and TNF-α showed inconsistent changes, likely due to differences in assay timing, participant fitness levels, and exercise modality. Despite some benefits in reducing soreness and preserving force production, omega-3 supplementation has not consistently improved range of motion (ROM) following eccentric exercise. Most included studies report no significant difference between groups [46]. Some studies reported that omega-3 supplementation may modulate oxidative stress markers, including reductions in malondialdehyde (MDA) and increases in endogenous antioxidant enzyme activity such as superoxide dismutase (SOD) and glutathione peroxidase (GPx). However, these effects were not consistently observed across all trials. Despite some reductions in subjective soreness, several studies failed to demonstrate significant changes in creatine kinase levels following omega-3 supplementation, indicating a possible dissociation between perceptual and biochemical outcomes.
Table 2 summarizes selected clinical studies examining their efficacy in reducing exercise-induced muscle damage
Table 2. Summary of Key Clinical Trials on Omega-3 Supplementation and EIMD
| Study | Population | Dose (EPA+DHA) | Duration | Exercise | Outcome |
| Tsuchiya et al. 2019 | Healthy men, untrained | 860 mg/day | 8 weeks | Biceps eccentrics | ↓ DOMS, ↑ ROM, ↓ MVC loss |
| VanDusseldorp et al. 2020 | Trained athletes | 2–6 g/day | 7 weeks | Eccentric squats | ↓ DOMS only in 6g group; no MVC or sprint benefit |
| Loss et al. 2022 | Healthy women, trained | 3.2 g/day | 3 days | Eccentric knee extensions | No significant change in DOMS or strength |
| Ramos-Campo et al. 2020 | Amateur endurance athletes | 2.1 g DHA + 240 mg EPA | 10 weeks | Squats + jumps | ↓ DOMS, ↓ LDH, ↓ IL-1β, ↑ recovery |
| Heileson et al. 2024 | Recreationally active | 4 g EPA or 4 g DHA | 7 weeks | Resistance training | EPA/DHA reduced DOMS, combo ineffective |
| Mackay et al. 2023 | Trained young males | 3 g/day | 4 weeks | Eccentric arm work | No effect on CK, soreness, or performance |
| Lewis et al. 2015 | Male athletes | 1.1 g/day (seal oil) | 21 days | Functional tests (jump, EMG) | ↑ EMG, ↓ fatigue, improved jump recovery |
| Kyriakidou et al. 2021 | Healthy untrained males | 3 g/day | 4 weeks | Downhill running | ↓ DOMS at 24h, no change in CK or IL-6 |
The effectiveness of omega-3 supplementation in reducing the severity of DOMS appears to be highly dependent on both dosage and duration of intake. Clinical trials have demonstrated that daily supplementation with 2 to 6 grams of combined EPA and DHA over a period of at least four weeks leads to the most consistent benefits, particularly in reducing soreness and limiting biochemical signs of muscle damage such as elevated creatine kinase and myoglobin levels [51, 52, 58]. Shorter protocols, especially those lasting fewer than five days, have generally failed to produce significant effects, likely due to insufficient incorporation of omega-3 fatty acids into muscle cell membranes and suboptimal modulation of inflammatory mediators [53]. Furthermore, higher doses tend to show stronger effects than lower ones, and some trials have even reported that EPA alone may be more effective than a combination of EPA and DHA, possibly due to differing pharmacokinetics and competing receptor activity [29]. The requirement for prolonged supplementation may reflect the time needed for EPA and DHA to displace pro-inflammatory fatty acids from cell membranes and initiate downstream signaling changes. This underlines the importance of designing protocols that ensure sufficient duration for therapeutic incorporation [44]. The anti-DOMS efficacy of omega-3 supplementation may be more evident in untrained individuals, who tend to exhibit greater inflammatory responses and sarcolemmal disruption following eccentric exercise. This suggests that training status may moderate the effectiveness of supplementation protocols. Notably, no clear dose-response relationship was observed in the included trials. Both low (e.g., <1 g/day) and high (>3 g/day) dosages of combined EPA and DHA have yielded mixed outcomes, highlighting the potential influence of other variables such as supplement composition and subject characteristics. Interestingly, a clear dose-response relationship has not been established. Both low (<1 g/day) and high (>3 g/day) dosages have shown variable efficacy, indicating that other factors—such as training status, supplement quality, and EPA:DHA ratio—may play a more decisive role in clinical outcomes [46].
Omega-3 supplementation shows the greatest efficacy in individuals with a lower baseline level of training or limited prior exposure to eccentric muscle loading. Untrained or recreationally active persons appear to exhibit a stronger inflammatory and structural muscle response to unfamiliar exercise, which makes them more susceptible to EIMD and more responsive to the protective effects of omega-3 fatty acids [30, 34]. In contrast, well-trained athletes, who typically possess muscular adaptations such as stronger sarcomeres, more efficient calcium handling, and attenuated cytokine responses, are less likely to benefit from omega-3 supplementation in the context of DOMS [59]. Nonetheless, certain subgroups of athletes, such as those entering high-volume training periods, performing eccentric overload routines, or undergoing rehabilitation after injury, may still experience tangible benefits. Furthermore, individuals with poor dietary intake of omega-3—such as those consuming minimal oily fish—may be particularly good candidates for supplementation regardless of training status. Omega-3 fatty acid supplementation at 4 g/day for four weeks significantly reduced delayed-onset muscle soreness and creatine kinase levels following high-intensity interval cycling in overweight and obese men. The supplementation also accelerated strength recovery compared to placebo, indicating particular benefit in populations with elevated baseline inflammation. Recent evidence indicates that omega-3 PUFA supplementation may yield enhanced benefits in populations with elevated baseline inflammation, such as overweight or obese individuals. For example, in a randomized controlled trial, supplementation with 4 g/day of EPA+DHA over 4 weeks significantly reduced DOMS and creatine kinase levels after high-intensity interval cycling in overweight men, and accelerated recovery of muscle strength compared to placebo [61].
Omega-3 fatty acids should not be viewed as a standalone solution to post-exercise recovery but rather as one component of a multimodal strategy. Evidence suggests that omega-3 supplementation may act synergistically with other recovery-enhancing agents such as high-quality protein, branched-chain amino acids, or creatine monohydrate, particularly in the context of strength training and rehabilitation [57]. Additionally, omega-3 can serve as a safe and potentially effective alternative to NSAIDs for individuals with gastrointestinal or cardiovascular contraindications, offering a way to modulate post-exercise inflammation without the risks associated with long-term NSAID use [9, 10]. Interestingly, the timing of omega-3 ingestion may also play a role, with some data suggesting that pre-exercise supplementation could be more effective than post-exercise use in blunting soreness and improving muscle function, although further research is needed to confirm this hypothesis [62]. The benefits of omega-3 supplementation appear to be more pronounced in untrained individuals, who exhibit a greater inflammatory and structural response to eccentric exercise compared to trained counterparts [46]. Recent evidence suggests that isolated supplementation with either EPA or DHA may yield superior effects on muscle recovery compared to combined formulations. In a double-blind randomized trial, supplementation with 4 g/day of either EPA or DHA over 7 weeks improved recovery markers after eccentric exercise, whereas the combined EPA+DHA group showed lesser effects. This suggests that these fatty acids may act via distinct and potentially competing mechanisms. Omega-3 fatty acid supplementation at 4 g/day for four weeks significantly reduced delayed-onset muscle soreness and creatine kinase levels following high-intensity interval cycling in overweight and obese men. The supplementation also accelerated strength recovery compared to placebo, indicating particular benefit in populations with elevated baseline inflammation [61]. Recent evidence suggests that isolated supplementation with either EPA or DHA may yield superior effects on muscle recovery compared to combined formulations. In a double-blind randomized trial, supplementation with 4 g/day of either EPA or DHA over 7 weeks improved recovery markers after eccentric exercise, whereas the combined EPA+DHA group showed lesser effects. This suggests that these fatty acids may act via distinct and potentially competing mechanisms. Notably, emerging data suggest that isolated supplementation with either EPA or DHA may be more effective than combined formulations. In a double-blind trial, 7-week supplementation with either 4 g/day of EPA or DHA produced superior recovery outcomes after eccentric resistance exercise compared to a group receiving combined EPA+DHA, possibly due to competitive inhibition at enzymatic or receptor sites [63].
In addition to monotherapy with omega-3 fatty acids, emerging research supports a synergistic approach combining omega-3 PUFA with other ergogenic or recovery-enhancing nutrients. A recent randomized controlled trial investigated the acute effects of omega-3 (1 g EPA+DHA) co-administered with 30 g of whey protein isolate in trained female futsal players undergoing high-intensity eccentric resistance exercise. The study revealed that the combined supplementation significantly attenuated DOMS, preserved muscle strength, and improved peak power output compared to placebo and single-nutrient interventions. Notably, the benefits were more pronounced when the supplementation was taken prior to exercise, suggesting a pre-conditioning effect against exercise-induced muscle damage [1, 62]. The proposed mechanisms underlying this synergy involve both nutritional components: whey protein enhances muscle protein synthesis through mTOR pathway activation , while omega-3 PUFA reduces inflammation, stabilizes cellular membranes, and modulates nociception. Together, they may facilitate a more efficient anabolic and anti-catabolic environment, promoting faster recovery and greater performance preservation. In addition, omega-3 PUFA may enhance the incorporation of amino acids into muscle tissue by improving cell membrane fluidity and insulin sensitivity, further potentiating the anabolic effects of protein [2][64]. These findings provide a rationale for the development of multimodal recovery protocols that integrate omega-3 PUFA with high-quality protein sources, particularly in elite or resistance-trained athletes exposed to repeated bouts of eccentric loading. Future studies are warranted to define optimal dosing, timing, and the potential additive effects with other micronutrients (e.g., vitamin D, antioxidants). In addition to the anabolic effects of protein co-ingestion, omega-3 PUFA may enhance insulin sensitivity and amino acid uptake, further supporting muscle protein synthesis. This dual action supports the rationale for co-supplementation in both resistance training and rehabilitation settings [62, 64].
Although omega-3 supplementation is widely regarded as safe, particularly at daily doses below 6 grams, several clinical considerations should be kept in mind. The most relevant safety concern is a potential increase in bleeding risk, particularly in individuals concurrently taking anticoagulants, antiplatelet agents, or NSAIDs [15]. This risk, while minimal in the general population, may be relevant in elite sport settings involving contact or injury risk. Some individuals also report gastrointestinal symptoms such as nausea or fishy aftertaste, especially when using lower-quality or poorly purified omega-3 products. Therefore, it is advisable to choose supplements that are third-party tested for purity, potency, and oxidation status, ideally bearing certification from reputable organizations such as IFOS or GOED.
Based on current evidence, the most practical and evidence-based approach for athletes and clinicians is to implement omega-3 supplementation as a preventive rather than reactive strategy. Initiating supplementation with 3 to 6 grams of EPA+DHA daily for a minimum of four to eight weeks appears to offer the most consistent benefits in reducing DOMS and improving recovery. This strategy is particularly relevant during the beginning of new training cycles, the off-season hypertrophy phase, or following a return to physical activity after injury or detraining. For individuals with chronic low fish intake or in situations where intense eccentric exercise is anticipated, lower maintenance doses of 1 to 2 grams daily may also be appropriate. Ultimately, the use of omega-3 supplementation should be individualized based on training load, recovery capacity, medical history, and dietary habits, and always considered as part of a broader recovery and performance plan.
Table 3 provides a practical guide for implementing Omega-3 fatty acid supplementation in athletic contexts.
Table 3. Suggested Protocol for Athletes
| Context | Protocol |
| Start of a new training cycle | 3 g/day EPA+DHA for at least 6–8 weeks |
| During heavy eccentric loading | 4–6 g/day for high soreness risk athletes |
| Recovery from injury or rehab | 2–3 g/day for 8+ weeks |
| Preventive low-level support | 1–2 g/day chronically (especially low-fish diets) |
Notably, the table underscores the importance of individualization, as optimal dosing may vary based on training load, dietary background, injury history, and responsiveness to Omega-3 supplementation.
The following conclusions can be drawn from the current body of evidence regarding the use of omega-3 polyunsaturated fatty acids in the context of EIMD:
Conceptualization: Justyna Jachimczak;
Methodology: Sebastian Kupisiak, Justyna Matusik;
Software: n/a; check: Piotr Rzyczniok, Filip Grydź;
Formal analysis: Aneta Rasińska, Justyna Jachimczak, Aneta Rostkowska, Mateusz Kopczyński;
Investigation: Joanna Filipow, Paulina Bala, Natalia Pasierb, Mateusz Kopczyński;
Resources: Aneta Rostkowska, Justyna Matusik;
Data curation: Aneta Rasińska, Sebastian Kupisiak;
Writing -rough preparation: Aneta Rasińska, Natalia Pasierb;
Writing -review and editing: Justyna Matusik, Mateusz Kopczyński;
Visualization, Justyna Matusik, Justyna Jachimczak, Paulina Bala;
Supervision: Aneta Rasińska, Paulina Bala;
Project administration: Piotr Rzyczniok.
All authors have read and agreed with the published version of the manuscript.
The authors declare no conflict of interest.