- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 14; 3. DOI 10.35630/2024/14/3.336
We conducted an experimental study on the effectiveness of the combined use of osteoplastic material Collapan-L with adhered MSCs to improve the processes of jaw bone regeneration in rats. The animals were divided into two groups: experimental and control groups of 50 animals each. Bone damage was induced in rats by drilling a defect with a dental bur in the area of attachment of the masticatory muscles in the angle of the mandible. We used only Collapan-L in the control group, and Collapan-L with adherent MSCs in the experimental group. During the cultivation process, statistically significant changes in concentration, depending on the day of cultivation, were observed for all cytokines: IL-1β (p<0.001 ANOVA), IL-2 (p=0.007 ANOVA), IL-4 (p=0.02 ANOVA), IL-6 (p=0.003 ANOVA), IL-8 (p=0.003 by Kruskal-Wallis test) and TNF-α (p<0.001 ANOVA). The dynamics of changes in the cytokines had a wave-like character. By the day 30 of the experiment, in animals with injected Collapan-L, the total volume of bone tissue in the regeneration zone was 50.14%, and in the Collapan-L + MSCs group it was 75.4%. The use of osteoplastic material Collapan-L with adhered MSCs is an effective combination for improving regeneration processes and reducing the inflammatory response of bone tissue.
Keywords: mesenchymal stem cells, bone regeneration, maxillofacial surgery, reparative osteogenesis, osteoplastic material
Despite a large number of Russian and foreign research works, there is a general agreement that the treatment of impaired post-traumatic reparative osteogenesis and bone tissue defects still remains an urgent problem, for which a number of aspects need to be clarified. Recently, the term “regenerative medicine” has become increasingly used in periodical literature. It implies the possibility of stimulating the restoration of the lost function of a particular organ [1-4]. This is due to the achievements of modern biology and medicine in the study of stem cells (SC), which provide great opportunities for the development of cell and tissue transplantation in various pathological processes [5-8].
Reparative bone regeneration consists of two processes: the first is the processes of bone destruction and the second is regeneration [9,10]. Identification of priority methods for influencing cellular differons responsible for reparative osteogenesis will speed up the process of bone tissue restoration [11-13].
Bone tissue is a derivative of mesenchyme and belongs to renewing tissues, which differon organization is represented from stem to highly differentiated cells. During both physiological and reparative regeneration, the precursors of osteogenic elements are mesenchymal stem cells (MSCs), which, under certain conditions, have the ability to differentiate according to the osteoblastic type [14,15].
A large number of experimental studies have convincingly shown that during both physiological and reparative regeneration, the precursors of osteogenic elements are mesenchymal stem cells (MSCs), which, under certain conditions, have the ability to differentiate according to the osteoblastic type [16]. MSCs isolated from the bone marrow adhere to the surface of culture flasks, proliferate and form colonies of fibroblast-like cells. These cells are capable of long-term self-support, and under the influence of certain inducers, they are capable of differentiation into osteoblastic type cells. During the cultivation process, MSCs synthesize elements of the fibrous intercellular substance which accumulate mineral components, forming foci of mineralization [17, 18]. By varying in vitro culture conditions, bone, cartilage and fibrous connective tissue, adipocytes and smooth myocytes are obtained from MSCs. Along with experimental work, data on the use of MSCs in clinical practice has increasingly began to appear in the literature. There are reports of the successful use of MSCs in the treatment of aseptic necrosis of the femoral head, osteochondral defects of the bones of the knee joint, false joints, nonunion and defects of long bones of the extremities [19, 20]. These data indicate that cell technologies have moved beyond experimental laboratories and are being actively introduced into clinical practice.
The purpose of the study was to investigate the effectiveness of the combined use of osteoplastic material Collapan-L with adhered MSCs to improve the processes of jaw bone regeneration in rats.
Bone marrow (BM) was taken under aseptic conditions from the breast bone (sternum) or from the iliac crest (crista iliaca) of 50 Wistar rats (experimental group) in an amount of 20-40 ml with the addition of 625 units/ml of heparin (Darnitsa, Ukraine). The BM aspirate was layered onto a Histopaque-1077 gradient, density 1.077 g/ml (Sigma, USA), and centrifuged for 30 min. at 1500 rpm. The resulting mononuclear cells were collected with a pipette and washed sequentially 3-4 times in Hanks' solution (Biolot, Russia) by centrifugation at 1000 rpm for 14 minutes. The resulting mononuclear suspension of bone marrow cells was seeded onto collagen-coated culture flasks with an area of 75 cm2 (Corning-Costar, USA) at a concentration of 2–5106 cells per flask.
MSCs were cultivated in a mixture of DMEM/F12 nutrient medium, 1:1 (Sigma, USA), with the addition of 10% fetal calf serum (Biolot, Russia), 0.75 mg/ml of glutamine (Institute of Poliomyelitis and Viral Encephalitis, Russia), 2 ng/ml of basic fibroblast growth factor (Sigma, USA) and 100 units/ml of penicillin and streptomycin (Darnitsa, Ukraine), in a CO2 incubator (Jouan, France) at 37ºС and 5% CO2 atmosphere. The medium was changed every 3-4 days of cultivation. The cultures reached the primary monolayer on days 8-11 of cultivation, depending on the seeding density of the initially isolated cell suspension, the individual characteristics of the donors and the level of cell proliferative activity.
Passaging, or subcultivation, was carried out using a mixture of trypsin/EDTA solutions (Biolot, Russia) in a ratio of 0.05%:0.02% in PBS, pH 7.4 (Sigma, USA). The passaging ratio was 1:2 or 1:3. After that the cells were cultured in a CO2 incubator under the same conditions. As a result of these manipulations, an uncommitted cell culture of MSCs was obtained.
After the cultures of uncommitted MSCs reached a confluent monolayer, they were incubated in an osteogenic growth medium containing DMEM/F12, 1:1, (Sigma, USA) with the addition of 10% fetal calf serum (Biolot, Russia), 0.75 mg/ml of glutamine (Institute of Poliomyelitis and Viral Encephalitis, Russia), and osteogenic supplement – 50 μg/ml of L-ascorbic acid (Sigma, USA), 10 mM of β-glycerophosphate (Sigma, USA), 0.1 μM of dexamethasone and 100 units/ml of penicillin and streptomycin (Darnitsa, Ukraine) in a CO2 incubator (Jouan, France) at 37ºС and 5% CO2 atmosphere. The growth medium was changed every 3-4 days of cultivation.
Bone damage was induced in rats by drilling a defect with a dental bur in the area of attachment of the masticatory muscles in the angle of the mandible. For a biocomposite material, we used Collapan-L (Intermedapatit LLC, Russia) which is an artificial hydroxyapatite, collagen, and an antimicrobial agent (lincomycin hydrochloride, colloidal silver, gentamicin sulfate, metronidazole, claforan, rifampicin, dioxidin, isoniazid). Collapan-L granules were immersed in MSC culture for 12 hours and used to fill the bone defect. The control group (50 animals) received Collapan-L alone. The experiment took place in a certified laboratory in compliance with the “European Convention for the Protection of Vertebrate Animals Used for Experimental or Other Scientific Purposes” [Directive 2010/63/EU].
The analysis shows that during the cultivation process, statistically significant changes in concentration were observed for all cytokines (CK), depending on the day of cultivation: IL-1β (p<0.001 ANOVA), IL-2 (p=0.007 ANOVA), IL -4 (p=0.02 ANOVA), IL-6 (p=0.003 ANOVA), IL-8 (p=0.003 by Kruskal-Wallis test) and TNF-α (p<0.001 ANOVA). The dynamics of changes in the CK had a wave-like character. In this regard, in order to determine the presence of a particular trend in the production of IL, their concentration in supernatants by committed MSCs was studied. Table 1.
Table 1. Dynamics of changes in the concentration of cytokines in the supernatants of committed MSC cultures (±m, kg10-15/l10-3)
| Cytokine | Day
10 (n=30) |
Day
13 (n=30) |
Day
16 (n=30) |
Day
19 (n=30) |
| IL-1β | 43,7±8,2 | 30,3±0,5 | 66,2±3,7* | 51,2±2,9 |
| IL-2 | 13,8±1,1 | 10,9±0,8 | 15,9±1,1 | 15,8±1,2 |
| IL-4 | 9,2±1,4 | 21,7±1,4* | 6,2±1,4 | 18,9±1,1* |
| IL-6 | 279,1±21,8 | 275,5±7,3 | 303,1±7,3 | 241,2±3,9 |
| IL-8 | 1119,0±53,2 | 1311,2±197,2 | 976,2±113,0 | 825,0±18,1* |
| TNF-α | 11,7±0,6 | 18,4±0,5* | 11,2±0,5 | 5,2±0,3* |
Notes: * – statistically significant difference (p<0.05) in relation to the day 10; – statistically significant difference (p<0.05) in relation to the day 13; – statistically significant difference (p<0.05) in relation to the day 16
Histological examination revealed that the use of osteoplastic materials with adhered MSCs leads to a significant improvement in osteoplasty. Fig.1.
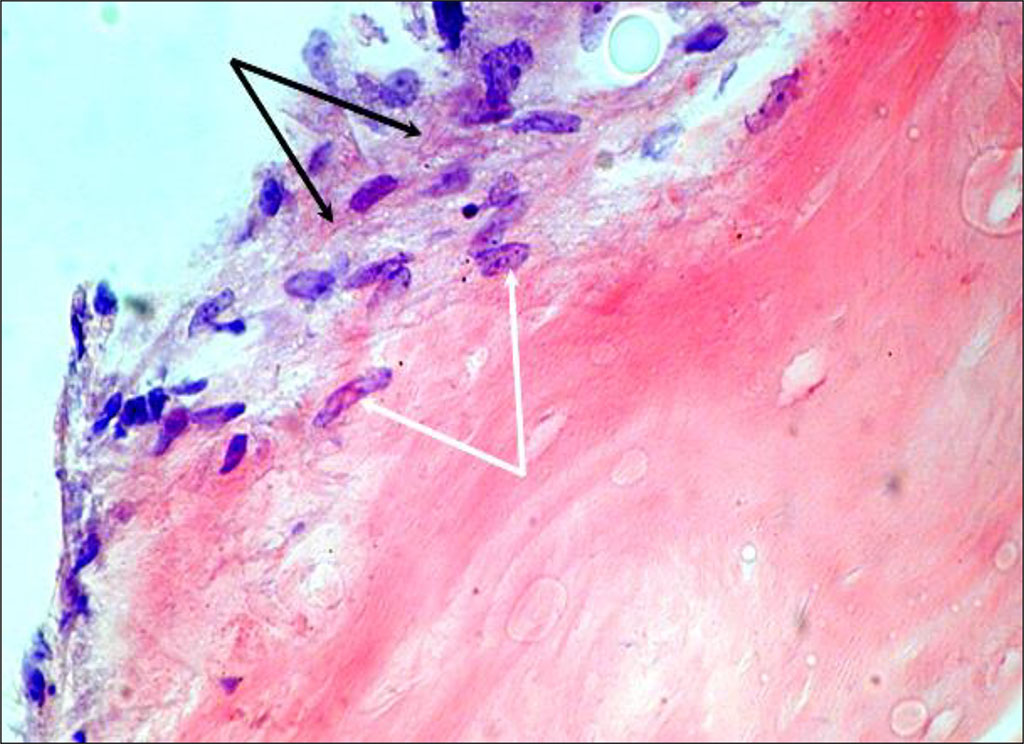
Figure 1. Cultured cells on Collapan-L. Day 7 of cultivation (staining with hematoxylin and eosin, magnification 400) – cell growth into the thickness of the carrier (white arrows) and formation of the cells’ own extracellular matrix (black arrows)
By the day 30 of the experiment, in animals injected with Collagen-L, the total volume of bone tissue in the regeneration zone is 50.14%, and in the Collapan-L + MSC group it is 75.4%.
The use of osteoplastic material Collapan-L with adhered MSCs is an effective combination for improving regeneration processes and reducing the inflammatory response. Committed MSCs actively produce cytokines: IL-1β, IL-2, IL-6, IL-8, TNF-α and IL-4. During the process of osteogenic differentiation, the production of IL-2, IL-6 and IL-8 significantly increases.