- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Introduction: In recent decades, the incidence of melanoma among European population has been steadily increasing. In addition, the age of patients with this pathology is declining - today more than half of patients with melanoma are under 65 years. Melanoma is an aggressive metastatic tumor that is difficult to treat. The tumor is resistant to chemotherapy and radiotherapy. However, the mechanism of photodynamic action differs from the mechanism of cell damage by antitumor drugs or ionizing radiation. Therefore, there is a high probability that with the help of photodynamic therapy it will be possible to overcome the resistance of melanoma cells to damage.
Materials and methods: The study was performed on melanoma tumors of B16 mice. The due methylene blue (MB) was used as a photosensitizer through the known affinity of this colorant to the melanin pigment of melanoma cells. The experiments used the radiation of two semiconductor lasers (Fotonika Plus, Cherkasy. Ukraine) with a wavelength of 660 nm, which corresponds to the maximum absorption of methylene blue.
Results:The possibility of increasing the anti-melanoma photodynamic activity of MB with the help of a modifier - the natural polysaccharide chitosan was studied. B16 melanoma has been shown to be sensitive to PDT with MB and its sensitivity can be increased by using MB with the natural polysaccharide chitosan, which has immunoadjuvant properties.
Conclusion: As a result of the presented research, a variant of PDT of B16 melanoma of mice was developed using MB sensitizer and chitosan immunoadjuvant, which includes intratumoral injection of MB together with chitosan, irradiation of tumors with a red laser with a wavelength of 660 nm, as well as the use of chitosan as an immunomodulatory factor.
Keywords: laser irradiation, photodynamic therapy, photosensitizer, melanoma, survival.
Melanoma is a malignant neoplasm resistant to chemotherapy and radiotherapy. [1,2] Research data show that melanoma is resistant to the most widely used chemotherapeutic drugs, in particular to doxorubicin. [3] However, the mechanism of photodynamic action differs from the mechanism of cell damage by antitumor drugs or ionizing radiation. Therefore, there is a high probability that with the help of photodynamic therapy it will be possible to overcome the resistance of melanoma cells to damage. [4-6]
In recent years, a number of in vitro and in vivo experimental studies have been performed on photodynamic effects on melanoma cells in culture and on mouse-inoculated tumors [7-9]. Photodynamic therapy induced apoptosis and autophagy in melanoma cells, arresting tumor growth, and prolonging the life of mice with grafted tumors, but complete remission was very rare. In clinical studies, regression of skin metastases of melanoma after PDT and the absence of side effects were obtained [10]. Antitumor effects consisted of direct damage to tumor cells, destruction of blood vessels in the tumor and activation of the immune response [11,12].
Regarding the choice of photosensitizer (FS) for photodynamic effects on experimental melanoma, the dye methylene blue (MB) is of interest. MB has been shown to accumulate only in melanin-containing cells [13].
Methylene blue belongs to the group of phenothiazine dyes. The MB molecule has a high redox potential. Depending on the conditions, MB can act as both an oxidant and a reducing agent. It is characterized by photochemical reactions of both types: the first type - with the formation of free radicals, and the second - with the formation of singlet oxygen [14].
As one of the possible modifiers of the photodynamic activity of MB, the natural polysaccharide chitosan attracts attention. Chitosan is a product of deacetylation of chitin, the external skeleton of crustaceans, insects and fungi. It is non-toxic, biocompatible, approved for use in pharmacology. Chitosan is a polycationic electrolyte, its molecule has many amino groups, so it easily forms complexes with negatively charged molecules, including FS molecules. In the biological environment, chitosan gradually degrades, releasing the attached molecules, so it is used in studies on the controlled release of drugs [15,18].
The MB molecule is positively charged, so chitosan does not form complexes with MB. But chitosan molecules have high mucoadhesiveness, as a result of which chitosan significantly facilitates the penetration of drugs into biological tissues. It has been shown that Chitosan reduces the activity of matrix metalloproteinases (MMPs), enzymes that provides invasion and metastasize tumors. There are data in the literature on the effect of chitosan on the activity of MMP-2, MMP-4 and MMP-9 [16,17].
The effect of chitosan on the activity of MMP-2 is especially important, because the substrate for this matrix metalloproteinase is type IV collagen, the main structural material of the basement membrane of the skin. Decreased chitosan activity of this metalloproteinase may inhibit melanoma invasion and metastasis
The aim of this study was to develop a variant of photodynamic therapy of B16 melanoma in mice using FS methylene blue and a modifier of its photodynamic activity - the natural polysaccharide chitosan.
The research was conducted on mice obtained from the vivarium of the Institute of Experimental Pathology, Oncology and Radiobiology named after R. E. Kavetsky of the National Academy of Sciences of Ukraine. The animals were kept in accordance with the "Standard rules for the organization, equipment and maintenance of experimental biological clinics (vivariums)". When working with animals, all requirements of the "European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes" (Strasbourg, 1986), the basic rules of good laboratory practice GLP (1981), the Law of Ukraine No. 3447-IV dated 21.02.2006 were observed "On the protection of animals from cruel treatment." Chitosan from Aldrich (Germany) and Kalipsovet (Ukraine; Category - Anti-stress and sedative drugs for veterinary practice - (50 mg ketamine/ml) and pharmacopoeial 1% solution of methylene blue (MS) in 0.9% solution were used in the work. NaCl. The injection volume varied from 50 to 100 μl depending on tumor size. The optimal concentration of MS was 10 μg/ml (1% solution), so 0.5-1 mg of MS, or 25-50 mg per 1 kg of body weight of mice, was injected into the tumor. Chitosan was dissolved in a 0.1% solution of acetic acid. Its concentration was 1-2 mg/ml. In the experiments, radiation from two semiconductor lasers (Photonics Plus, Cherkasy, Ukraine) with a wavelength of 660 nm, which corresponds to the maximum absorption of methylene blue, was used. Melanoma strain B16 was obtained from the IEPOR human and animal tissue cell bank. Laser irradiation doses ranged from 90 J/cm2 to 300 J/cm2 at different power densities - from 75 mW/cm2 to 250 mW/cm2. Irradiation exposure 20 min. The variation in the parameters of animal irradiation was caused by the determination of the optimal dose of laser irradiation. To examine differences between groups, one-way analysis of variance was performed using the OriginLab program. Differences between values with p<0.05 were considered significant. Values were expressed as mean ± standard deviation (SD).
When determining the sensitivity of experimental melanoma to different doses of laser radiation, a number of doses of laser light were tested - from 90 J / cm2 to 300 J / cm2 at different power densities - from 75 mW / cm2 to 250 mW / cm2/ Figure 1 shows the duration of life of mice with B16 melanoma in the experiment, which used two radiation doses - 180 J / cm2 and 360 J / cm2 at the same power density - 150 mW / cm2.
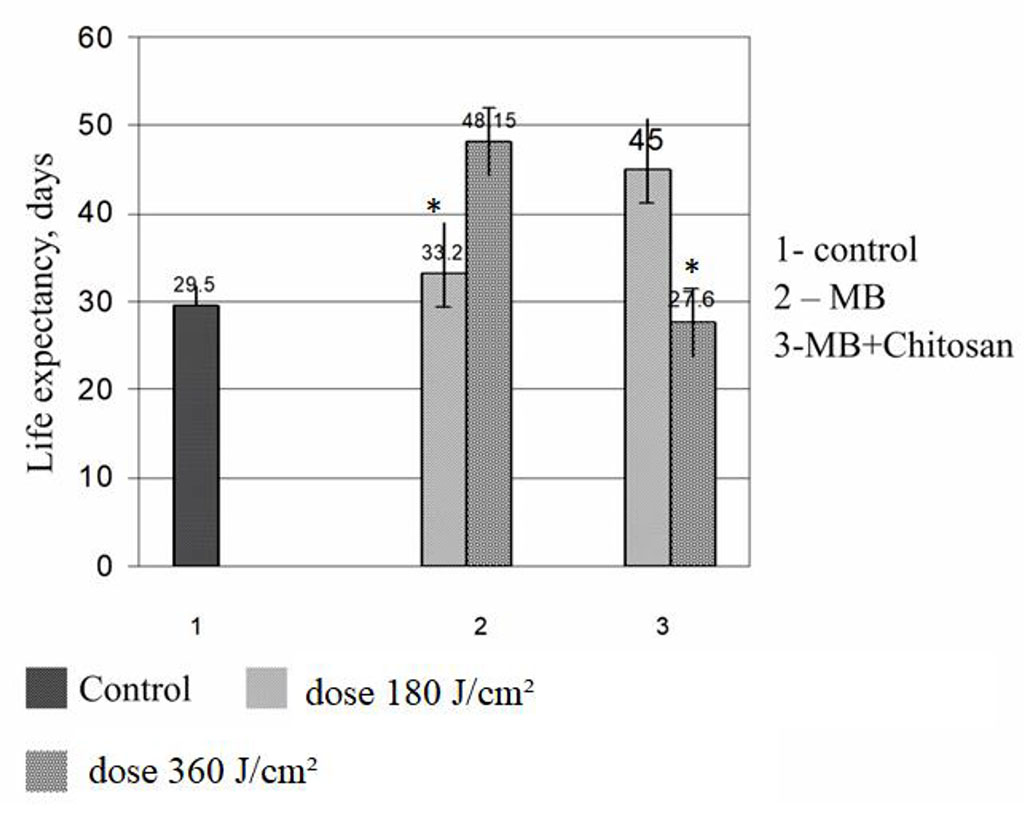
Figure.1. Life expectancy of mice with B16 melanoma after photodynamic therapy with MB and with MB + chitosan. *p< 0,05 180 vs 360
The figure shows that animals that received MB with chitosan and were irradiated with a light dose of 360 J / cm2, died at the same time as the mice of the control group, or even a little earlier. Therefore, in subsequent experiments, this dose of radiation was abandoned. In the group where animals received MB and were irradiated for 20 minutes, the life expectancy was also not significantly different from the life expectancy of the control mice.
Figure 2 shows the dynamics of tumor growth in the control group of mice and mice that did not survive in other groups after photodynamic exposure. It should be emphasized that in other experiments performed by us on photodynamic therapy of B16 melanoma, the nature of tumor growth in non-surviving mice was the same as shown in figure.
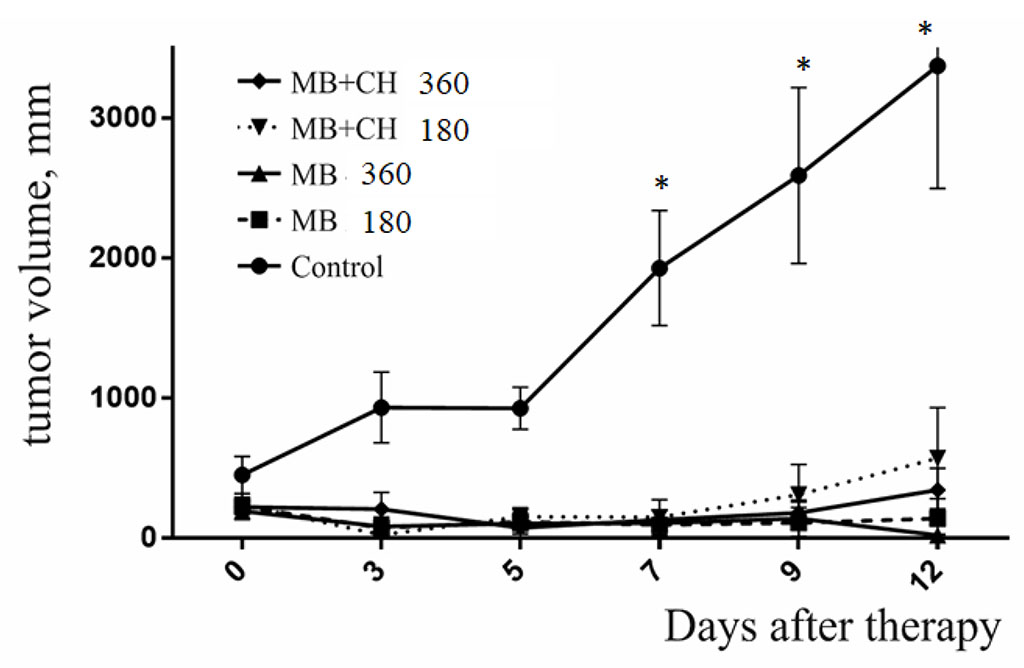
Figure. 2. Dynamics of B16 melanoma growth in mice that did not survived after photodynamic therapy with MB. *p< 0,05
Figure 3 presents the dynamics of death and survival of mice subjected to photodynamic therapy with methylene blue or methylene blue together with chitosan in the experiment described above.
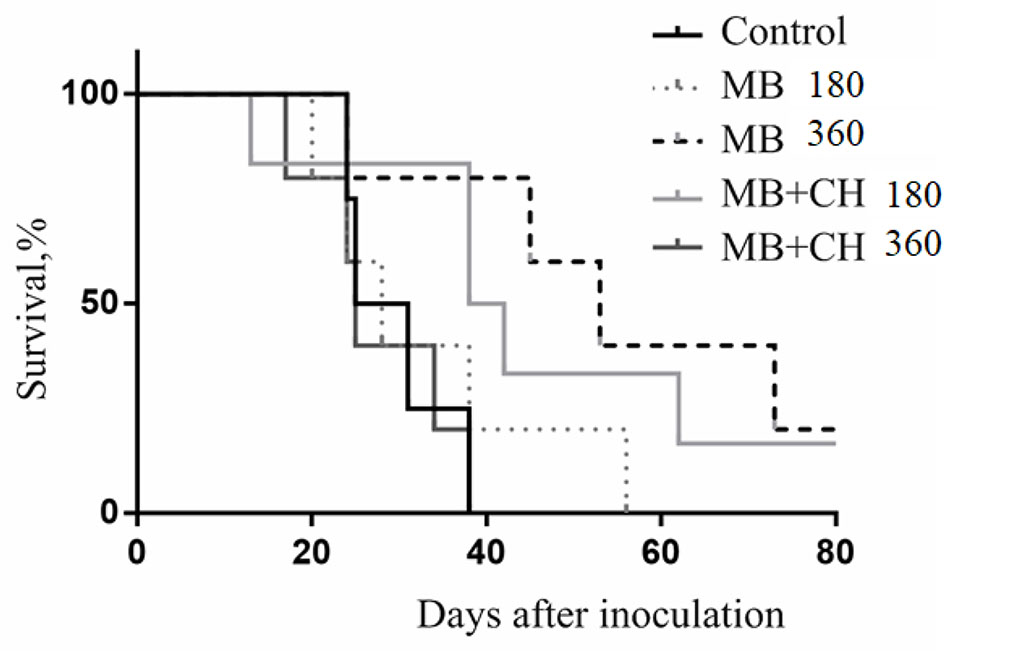
Figure. 3. Dynamics of death and survival of mice with B16 melanoma after photodynamic therapy.
More encouraging results were obtained in experiments using the natural polysaccharide chitosan as a modifier of photodynamic antitumor activity of MB. It is known from the literature that the natural polysaccharide chitosan, in addition to facilitating the delivery of drugs to target tissues, has a significant immunomodulatory effect [16,17]. In this case, intra-abdominal administration of chitosan causes activation of only humoral immunity, and subcutaneous- and humoral, and cellular (activation and proliferation of T-cytotoxic lymphocytes). In the next experiment, we used a laser radiation dose of 150 J / cm2 at a high power density of 250 mW / cm22.
In this experiment, half of the mice of the group survived, in which MB was injected into the tumor together with chitosan and additionally - chitosan subcutaneously (Fig. 4). In total, in experiments in which melanoma tumors were irradiated after intra-tumor administration of MB together with chitosan with additional subcutaneous administration of chitosan after irradiation, complete elimination of tumors and survival of mice was observed in 50% of cases. The lifespan of non-surviving mice in such experiments was one and a half to two times longer than in controls.
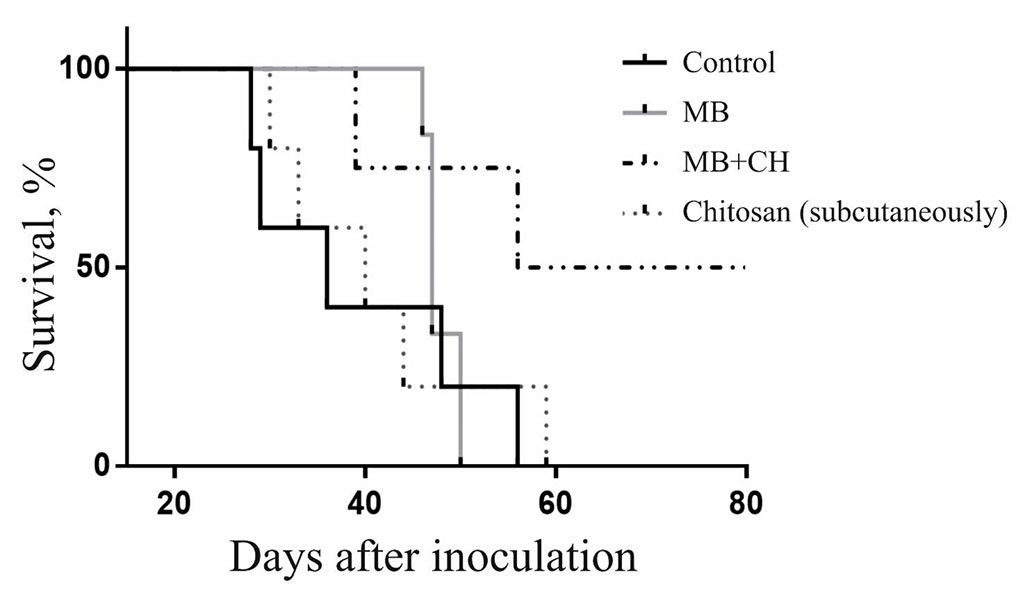
Figure. 4. Dynamics of death and survival of mice with B16 melanoma after photodynamic therapy with additional subcutaneous injection of chitosan.
A series of in vivo experiments on mice with B16 melanoma showed that this tumor is sensitive to photodynamic therapy using methylene blue as a photosensitizer. The possibility of strengthening the anti-melanoma photodynamic activity of MS with the help of a modifier - a natural immunosuppressant polysaccharide chitosan - was studied. With the combined use of MS with chitosan as an additional immunomodulatory agent, complete destruction of the tumor and survival of 50% of mice with B16 melanoma was achieved. In the rest of the mice that underwent photodynamic therapy, the lifespan increased by one and a half to two times compared to control untreated animals. The conducted research logically continues the trend of recent years towards the complex use of photodynamic therapy together with immunotherapy. In 2016 and 2018, two patents appeared from researchers in Norway [19,20], who created nanoconjugates of porphyrin photosensitizers (meso-tetra-phenylporphyrin and aminophenyl-triphenylporphyrin) with chitosan and tested them in in vitro experiments on the cell line. human colon HCT11 and in vivo in tumor-inoculated mice. They demonstrated an increase in the photodynamic activity of FS in relation to tumor cells and the stimulation of the immune response in irradiated mice.
Data from the literature show that in combination with photodynamic therapy, the effectiveness of doxorubicin, mitomycin, methotrexate, cisplatin, 5-fluorouracil, cyclophosphamide and gemcitabine has traditionally been studied the most. [21,24]. Importantly, PDT induces apoptosis and necrosis in treated tumors, causes damage to the microcirculatory tract and leads to inflammation, hypoxia and oxidative stress. These processes correlate with increased regulation of angiogenesis factors, such as factor - 1 (HIF - α), vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF - α), interleukin1β (IL-β), prostaglandin E2 (PEG2), cyclooxygenase - 2 (COX-2), servaivin and matrix metalloproteinases in PDT-treated tumors. The fact is that these regulatory molecules can provoke tumor recurrence. The combination of PDT with angiogenesis inhibitors and survival molecules may improve the prognosis. For example, a combination of COX-2 inhibitors, TNF-α inducers, or antiangiogenic agents may potentiate the PDT response and lead to more effective tumor suppression. Moreover, the use in synergy of modern photosensitizers, the latest systems of drug transport to the tumor - various nanoparticles, including the use of target markers, as well as monoclones - can improve the results of anticancer treatment and improve the quality of life of patients [22,23,25,26].
It is also important to note that, in addition to the development of photodynamic therapy, in recent years more and more good results have been achieved by laser phototherapy of low energy, in particular with the use of infrared light. The use of photobiomodulation to enhance the antitumor immune response and prevent complications of chemotherapy and radiation therapy seems promising [27,28]. Therefore, research in these areas is promising and should be continued.