- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
This research involves a comparative study of the immunohistochemical features of the inflammatory response of the anterior abdominal wall tissues after implantation of the autoderm and polypropylene mesh in rats. The object of the study were 36 Wistar rats, which were divided into two groups of 18 specimens each. In the first group, a polypropylene mesh was implanted; in the second group, de-epithelialized skin (autoderm) was implanted. On days 3, 7, 14, 30, and 90, we studied: CD68 as a pan-macrophage marker, CD3 as a pan-lymphocyte marker, and Bcl-2 the apoptosis regulator. Statistical analysis was performed using the Spearman-Rho-Test. The peak of an acute inflammatory response in the tissues after the implantation of polypropylene mesh and autoderm occurs 72 hours after the surgery. Arresting of acute inflammatory response after autodermal implantation is achieved 30 days after surgery. A specific reaction of the anterior abdominal wall tissues to polypropylene, in contrast to the autoderm, is the formation of a persistent chronic inflammatory response 90 days after the operation, which is due to the starting biodegradation of the synthetic implant. The Spearman-Rho-Test showed a highly significant correlation between CD68 and CD3 (r = 0.341, p = 0.001). A high correlation was found between CD68 and Bcl-2 (r = 0.195, p = 0.033) and CD3 and Bcl-2 (r = 0.220, p = 0.021). CD3, CD68 and Bcl-2 markers give evidence of the severity of the inflammatory response.
Keywords: polypropylene mesh, autoderm, implantation, inflammation markers, biodegradation, immunohistochemistry
Biocompatibility deals with phenomena that can be very multifaceted as they are influenced by many additional idiosyncratic factors, such as genetic, epigenetic including factors of viral origin, immunological, as well as complex mechanical, physical and pharmacological variables [1,2]
Lately, researchers have noted an increase in the number of patients requiring mesh removal due to a systemic reaction to the implant. Polypropylene, a widely used non-absorbable mesh material, has been found to degrade in vivo, which is undesirable for non-absorbable implants and contrary to biostability requirements. This inflammatory response to the implant material is called "Autoimmune/inflammatory syndrome induced by adjuvants (ASIA)" or "Shoenfeld's syndrome". There are works showing the development of ASIA in patients after mesh implantation, for example, after hernia repair or surgery for pelvic organ prolapse, as well as after silicone breast implantation ("breast implant illness"). It is currently unclear why a patient may develop Shoenfeld's syndrome. Some classify these systemic reactions to implants as mediated foreign body reaction, activation of systemic inflammatory markers in response to the implant, response to in vivo degradation and absorption of implants [3].
In connection with the above, recently, researchers have intensified their interest in bioimplants, including autografts, and specifically, autoderm. It is assumed that patient’s own tissue will not cause a pronounced local and general inflammatory response. The autograft will sprout with vessels and newly formed connective tissue, while it will maintain its strength and anisotropy properties [4–6]. At the same time, there are not enough studies on the features of the inflammatory response to autoderm.
The purpose of the study was to conduct a comparative study on immunohistochemical features of the inflammatory response of the anterior abdominal wall tissues after implantation of the autoderm and polypropylene mesh in rats.
All the studies were carried out in certified laboratories in compliance with the European Convention for the Protection of Vertebrate Animals used for Experimental or other Scientific Purposes [Directive 2010/63/EU]. The object of the study were 36 male Wistar rats weighing 200±50 g. The animals were divided into two equal groups of 18 specimens each. The surgery was performed under general anesthesia by an intraperitoneal injection of pentobarbital. In the first group, a polypropylene mesh prosthesis was implanted with a thread thickness of 0.60-0.68 mm and a density of 65 g/m2. The second group had the de-epithelialized skin (autoderm) implanted. Implantation was made in the retromuscular space of the anterior abdominal wall using the sub lay technique. The implants dimensions were 2.0x1.0 cm. Animals were removed from the experiment on days 3, 7, 14, 30, and 90 by decapitation under ether general anesthesia. We studied the inflammatory markers: CD68 as a pan-macrophage marker: murine monoclonal antibody (Roche Diagnostics GmbH, Mannheim, Germany), 1:200; CD3 as a pan-lymphocyte marker (PrimeBioMed, Russia), 1:200; rabbit antibodies to Bcl-2, SP66 clone (Roche, Switzerland), 1:200. An UltraVision Quanto Detection System HRP Polymer (ThermoFisher, USA) was used as a detection system in accordance with the manufacturer's instructions. We calculated the relative expression area (%) using the ImageScope program (Leica Biosystems, Austria). Cellular expression was assessed at four levels (negative - less than 5%, moderate - from 5% to 30%, strong - 30%-60% and pronounced - more than 60% of positive cells). Statistical analysis was performed using the Spearman-Rho-Test, which was used to test the correlation between each of the two factors, implantation time and cell expression. The r indicator describes the strength of the correlation. Statistical significance was assumed to be p < 0.05. Statistical analysis was performed using IBM SPSS Statistics for Windows version 28.0. (IBM Corp. USA).
When examining CD3 the panlymphocyte marker 72 hours after implantation, a pronounced and strong level of expression of CD3+ cells was noted in the first and second groups.
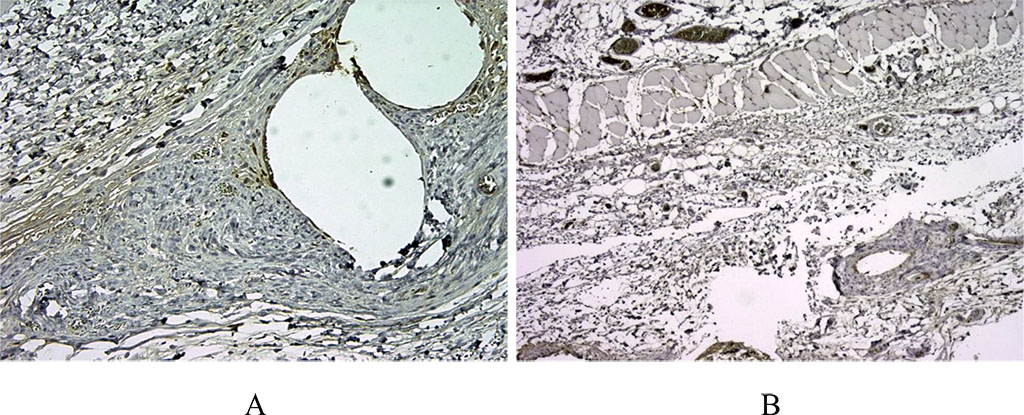
Figure 1. 14 days after implantation, a pronounced lymphoplasmacytic infiltration was noted in the first group. There are many CD3+ cells in this zone, about >60% of the inflammatory infiltrate around the voids – the locations of polypropylene monofilaments (A). In the second comparison group with the use of a bioimplant, we noted a weaker CD3 staining, which indicated a moderate inflammatory response (B). (X100).
There was no statistical difference in the indicators (p≥0.05). On the day 14 after the surgery, a different level of CD3+ expression was noted. Thus, in the first group, a pronounced level of expression of cells around the polypropylene implant was noted, while in the second group, a moderate level of expression was registered (p≤0.05).
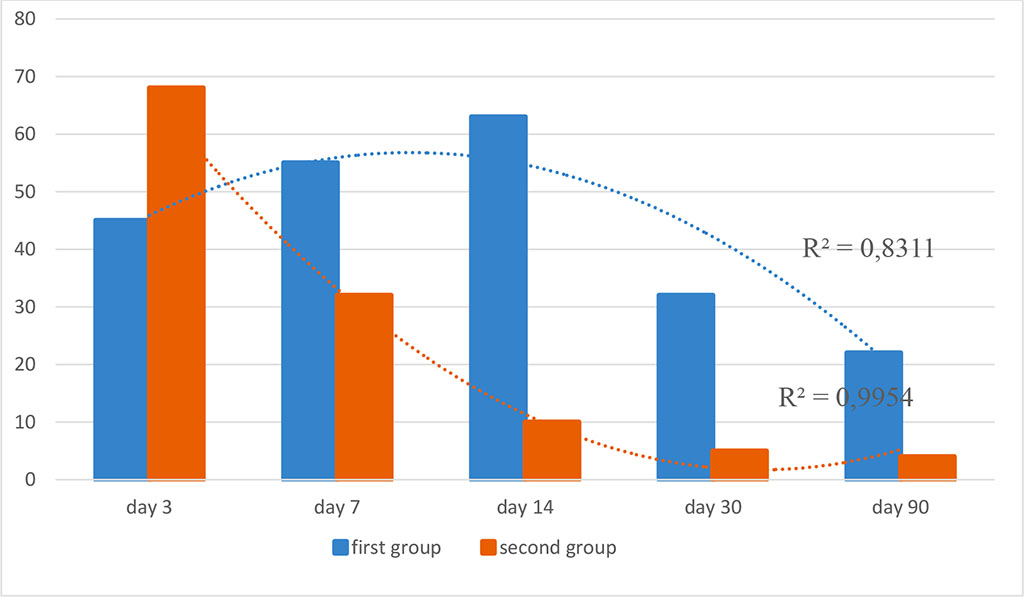
Figure 2. Dynamics of CD3+ lymphocytes in the study groups (optical density, %; R2 is the value of the reliability of the approximation)
Bcl-2 showed a strong degree of expression in both groups and did not differ statistically (p≥0.05).
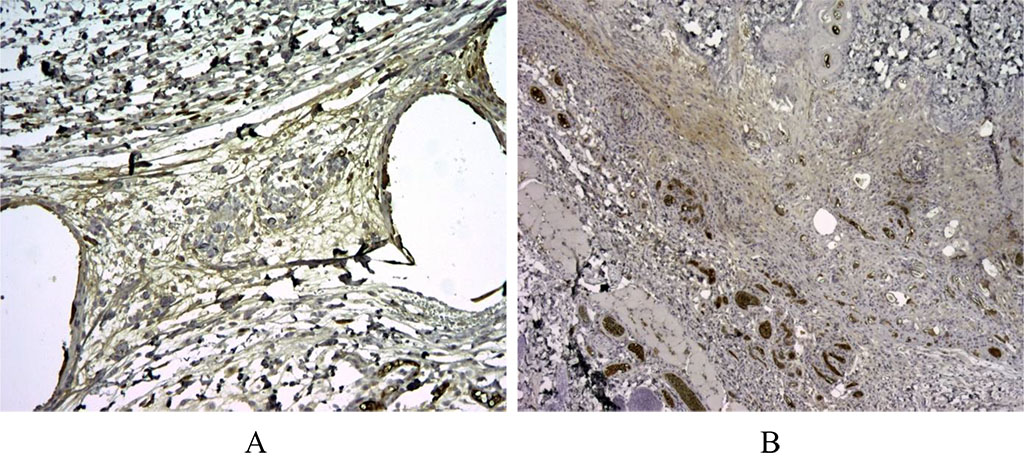
Figure 3. 30 days after implantation. When studying Bcl-2, the regulator of apoptosis, in the first group, specific staining was noted around the voids – the sites of implantation of the synthetic graft, as well as background staining on the vessels for Bcl-2+ (A). In the second group, specific Bcl-2+ staining was not noted (B). X100.
A distinctive trend was observed by the day 30 after implantation, when a pronounced expression level of Bcl-2+ cells was registered in the first group, and in the group with autodermal implantation, the expression level was non-existent and did not differ from the background level (p≤0.05).
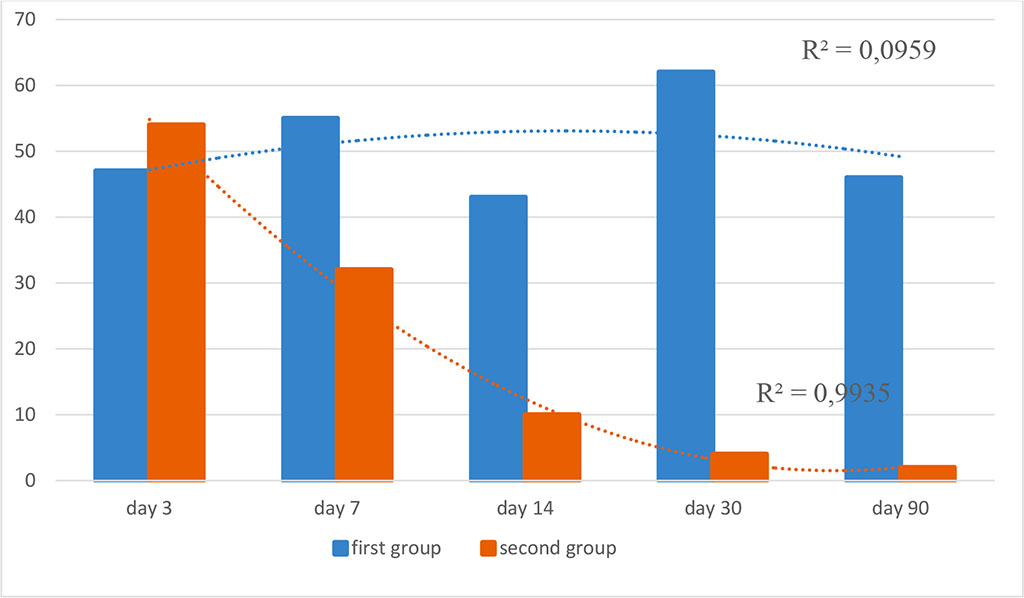
Figure 4. Dynamics of Bcl-2+ expression in the study groups (optical density, %; R2 is the value of the reliability of the approximation)
The study of CD68 showed that, on the day 3 after implantation, a pronounced level of expression was noted in the second group. At the same time, the macrophage reaction to the mesh was moderate and the optical density did not exceed 30% (p≤0.05).
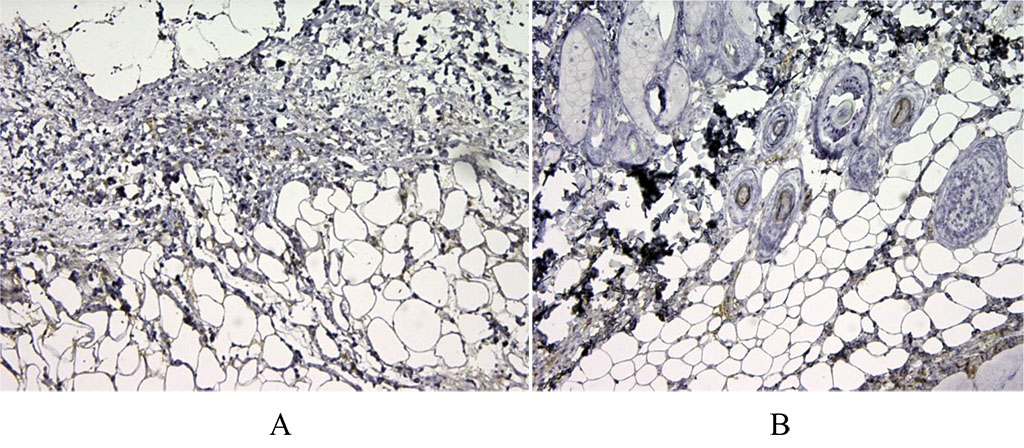
Figure 5. 3 days after implantation. We have noted moderate expression of CD68+ in the first group of animals (A). There is pronounced expression of the macrophage marker CD68 in the second group after autodermal implantation (B). X100.
Further, on the days 7, 14 and 30, the values did not have a statistical difference and were characterized by a moderate and strong reaction (p≥0.05). Another picture was noted on the day 90. In the mesh group, the macrophage reaction increased and was characterized as pronounced, while in animals with autoderm implantation, the level of CD68 expression was moderate (p≤0.05).
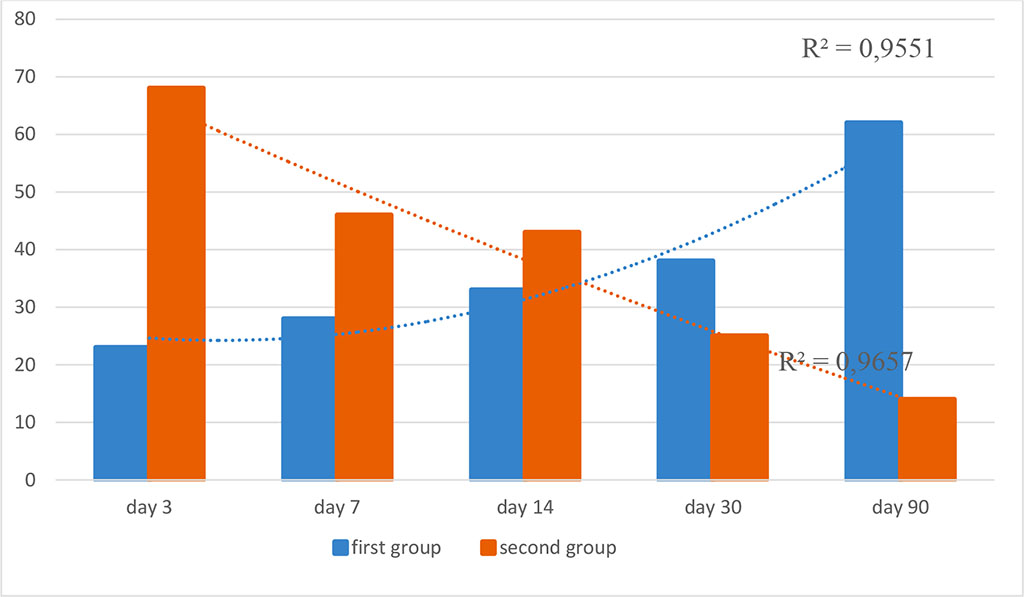
Figure 6. Dynamics of expression of CD68 panmacrophage marker in the study groups (optical density, %; R2 is the value of the reliability of the approximation)
Thus, after implantation of both mesh and autoderm, the acute inflammatory response of the tissues reaches its highest level after 72 hours, and then it gradually decreases to a harmless level. However, in the first group, there is no trend towards a decrease in the number of macrophage cells in the tissue of the anterior abdominal wall with time, as might be expected. There is a persistent inflammatory response induced by macrophages and neutrophilic granulocytes in the tissue, which is consistent with previous studies [19, 20]. This indicates the maintenance of immunity features that contribute to the persistence of inflammation and special tissue regeneration around the polypropylene mesh.
The result of this study indicates an increasing trend of chronic inflammatory response that may be caused by the starting mesh degradation. The terms of the start of mesh biodegradation in rats are unknown. In humans, according to recent studies, the terms are 5-10 years. Given the intensity of metabolism in rats, one should assume a much earlier start of biodegradation. A significant correlation of stronger CD68 expression points to persistent accumulation of macrophages, the central cells of the chronic inflammatory response, likely resulting from exposure to mesh biodegradation metabolites. In addition, stronger CD3 expression in the first group correlates with the severity of the inflammatory response. At the same time, in the second group, the dynamics of the expression of the anti-apoptotic marker Bcl-2 may indicate the relief of the inflammatory response by the day 30 after implantation. Although it should be noted that we did not come across any study of the Bcl-2 marker after polypropylene and autoderm implantation.
In addition to the correlation of inflammatory cells depending on the type of implant, there were significant correlations between CD68, CD3 and Bcl-2. This highlights the complex interaction between inflammatory cells in different phases of inflammation, which is consistent with the study by Klinge et al. [7] The Spearman-Rho test shows a highly significant correlation and more distinct cellular expression between CD68 and CD3 (r = 0.341, p = 0.001). A high correlation was found between markers CD68 and Bcl-2 (r = 0.195, p = 0.033) and CD3 and Bcl-2 (r = 0.220, p = 0.021).
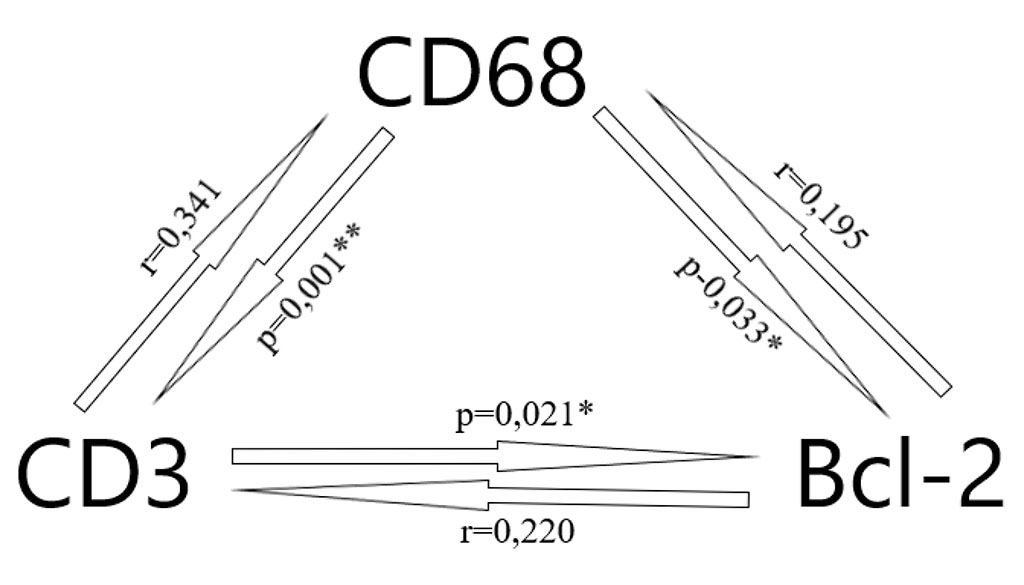
Figure 7. Correlation between the cells expression (* – p < 0.05, ** – p < 0.01)
The released microparticles, as a result of the polypropylene biodegradation, stimulate the accumulation of macrophages. Secreted highly concentrated reactive oxygen species cause oxidative stress and lead to further mesh degradation, damage to cells and DNA. The release of fine polypropylene particles into tissue can lead to health risks such as cytotoxicity and hypersensitivity due to increased levels of cytokines and histamines. The potential consequences of mesh degradation do not seem to be limited to the immune inflammatory response and therefore should not be overlooked in further studies [8-12].
To the best of our knowledge, this is the first study that analyzes the correlation of the inflammatory response of tissue after implantation of a polypropylene mesh and biotissue graft – an autoderm. Histological analysis in this study was limited to the most common inflammatory cell markers. The result shows that the implantation and subsequent biodegradation of the polypropylene mesh leads to an increased local inflammatory response in the tissues of the anterior abdominal wall in rats. Thus, modern materials for implantation should have high biostability, and the ability to integrate with tissues with a minimum inflammatory response in the surgical area. Autoderm has such properties, although there are some circumstances limiting the use of this method.
Further research on the effect of implants on various cellular activities, cytokine secretion, mechanical changes, microorganisms, etc. is necessary and urgent for the safety and well-being of patients.
The peak of an acute inflammatory response in the tissues after the polypropylene and autoderm implantation occurs 72 hours after the surgery. Relief of acute inflammatory response and postoperative apoptosis to autodermal implantation occurs 30 days after the surgery and remains at a minimal level during the study period. A distinctive feature of the reaction in the anterior abdominal wall tissues to polypropylene is the formation of persistent chronic inflammatory response 90 days after the surgery, which is caused by initating biodegradation of the synthetic implant. Immunohistochemical markers CD3, CD68 and Bcl-2 make it possible to assess the severity of the inflammatory response in tissues after implantation.