- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 3: e1. DOI 10.35630/2199-885X/2022/12/3.10
Aim: To compare the microvascular and metabolic effects of autologous blood and a balanced electrolyte solution (BES) containing acetate and malate in hemorrhagic shock (HS) in rats.
Methods. The experiments were carried out on male Wistar rats (n=21) under general anesthesia. Blood (35% of estimated blood volume) was withdrawn for 5 minutes. After 60 minutes of HS the collected blood was retransfused, followed by the infusion of BES in the volume of 50% of the blood loss (HS+AB group); HS+BES group received BES in a triple volume of blood loss; a sham-hemorrhage control. Heart rate (HR), mean arterial pressure (MAP), skin blood flow (M), gases and acid-base state of arterial blood, post-occlusive reactive hyperemia (PORH) in the rat tail, serum levels of biomarkers S100b and NSE were assessed after 24 hours. After euthanasia, a histological examination of the brain, lungs, heart and kidneys was performed.
Results. In HS+AB and HS+BES groups, 24-hour mortality did not differ (one animal in each group). MAP in these groups was moderately reduced compared to sham group. There were no significant differences in HR, M, acid-base state, PORH and levels of S100b and NSE between the groups. Histological examination revealed circulatory disorders of varying severity in groups with hemorrhage, with more pronounced damage to the brain and kidneys in HS+BES group.
Conclusion. Both autologous blood and BES containing acetate and malate equally restore MAP, skin microcirculation, and acid-base state 24 hours after HS. However, fluid resuscitation with BES alone is associated with brain and kidney damage.
Keywords: hemorrhagic shock, autologous blood, balanced electrolyte solution, rat, microcirculation, PORH, lactate, acetate, malate, brain damage
Fluid resuscitation is one of the key components of the treatment of patients with blood loss and hemorrhagic shock (HS). The composition and volume of fluids used in the complex of intensive care largely determine the effectiveness of the treatment of hemorrhage in severe trauma and other pathological processes [1, 2]. According to modern clinical guidelines, the following are the basic principles for fluid resuscitation in HS: start infusion using balanced crystalloid solutions; avoid excessive use of saline; limit the use of colloids due to their adverse effects on the hemostasis; maintain the target level of hemoglobin at 70-90 g/l; in the first hours after injury and blood loss, consider transfusion of fresh frozen plasma in a ratio of 1:2 to erythrocytes [3]. The latter statement is one of the components of the modern concept of Damage-Control Resuscitation, which aims to quickly stop bleeding, minimizing homeostasis disorders and preventing organ failure in the post-hemorrhagic period [4].
More and more publications indicate that in HS it is important to quickly restore not only systemic hemodynamics, but also microcirculation in order to correct or prevent subsequent organ dysfunction [5, 6]. Despite the fact that in preclinical studies, colloids and some blood substitutes have shown their effectiveness in restoring microcirculation [7], balanced electrolyte solutions (BES) and transfusion of donor blood components are currently the basis of fluid therapy for HS [1, 3]. It is important to evaluate the effectiveness of the infusion solution not only as the restoration of perfusion and tissue oxygenation, but also in terms of its effect on the parameters of the hemostasis (development of coagulopathy), the severity of organ failure, and treatment outcomes [8].
Both preclinical and clinical studies indicate the advantages of BESs over unbalanced ones when they are used to correct hypovolemia in blood loss or sepsis [6, 9]. Given these data, the focus in scientific research has shifted to comparing the efficacy and safety of BESs of different composition (for example, containing lactate, acetate or malate as a buffer base) [5, 10, 11].
The use of donor blood products for the correction of posthemorrhagic anemia is associated with a high risk of post-transfusion complications, especially with massive transfusions. In this regard, the technology of retransfusion of blood poured into the body cavities, and in particular, the transfusion of "washed" red blood cells, has acquired particular relevance [4]. Retransfusion of autologous blood in combination with judicious use of BES, can be considered an ideal strategy for replenishing blood loss [12].
The aim of this study was to compare the microvascular and metabolic effects of infusion of heparinized autologous blood and a BES containing acetate and malate in rat model of HS.
The experiments were carried out on male Wistar rats weighing 250-350 g (n=21) in accordance with the accepted international standards for working with laboratory animals [13] and after approval of the study protocol by the local Ethical Committee of the Federal Research and Clinical Center of Intensive Care Medicine and Rehabilitology.
Manipulations with laboratory animals were performed under general combined anesthesia: xylazine (Xylanit, NITA-PHARM, Russia) 5 mg/kg intraperitoneally and tiletamine/zolazepam (Zoletil 100, Virbac, France) 20 mg/kg intraperitoneally. The animal breathed spontaneously during the experiment.
For invasive measurement of blood pressure and sampling of arterial blood, the left carotid artery was catheterized with a PME-60/PE catheter, OD 0.6 mm, ID 0.3 mm of the intravascular part of the catheter (Sci Cat, Russia). For sampling of venous blood, infusion of blood and solutions, the right jugular vein was catheterized with a PME-60 catheter, OD 0.8 mm, ID 0.4 mm (Sci Cat, Russia). Catheters were flushed with a small portion of unfractionated heparin solution (20 U/ml) as needed.
Then the rat was fixed in the prone position on the heated platform of the MouseMonitor S monitor (INDUS Instruments, USA). The central body temperature was maintained at the level of 36.0-37.0oC. The period of stabilization of the animal before the start of measurements was 15–20 min.
A fixed-volume hemorrhage model was used [14]. The estimated blood volume (EBV) for the rat was 6.5% of body weight. The volume of blood loss was 35% of the EBV (23 ml/kg of body weight). Arterial blood was collected for 5 minutes into a sterile syringe containing 50 U of unfractionated heparin. Prior to reinfusion, the blood was stored at room temperature (22-25°C). The period of untreated HS lasted 60 minutes.
Animals were divided into three groups (n = 7 in each group). Group I – HS with retransfusion of autologous blood (HS+AB) over 10 minutes followed by infusion of a BES containing malate and acetate (Sterofundin isotonic, B.Braun Melsungen AG, Germany) over 10 minutes in the amount of 50% of the volume of blood loss. Group II – HS with infusion of the BES over 20 minutes in a triple volume of blood loss (HS+BES). Triple-volume infusion of crystalloids for fluid resuscitation in HS is often used in experimental research [10], due to their weak volemic effect (rapid redistribution from the vascular bed to the interstitium). Group III - sham-treated animals (Sham), subjected to all procedures (anesthesia, surgery and observations) except hemorrhage and infusions.
After reperfusion and recovery from anesthesia, the animal was transferred to a cage with free access to water and standard food. For the purpose of postoperative analgesia, paracetamol 30 mg/kg was administered subcutaneously. The next day (22-26 hours after hemorrhage) the animals were anesthetized again. After data recording, the animals were euthanized by intravenous administration of 1 ml of 4% KCl solution under general anesthesia and internal organs (brain, lungs, heart, kidneys) were withdrawn for subsequent histological analysis.
Heart rate (HR) and central body temperature were measured using the MouseMonitor S system (INDUS Instruments, USA). Invasive measurement of mean arterial pressure (MAP) was carried out through a catheter in the carotid artery using a BP-100 device (CWE, Inc., USA) and a Deltran DPT-100 transducer (Utah Medical Products, USA). Using a PowerLab16/35 analog-to-digital converter and LabChart Pro 8 software (ADInstruments, Australia), the average values of HR and MAP over 5 minutes were calculated.
Local blood flow (perfusion) in the skin of the tail was measured by laser Doppler flowmetry (LDF). The optical probe of the LAZMA MC-3 device (laser wavelength 1064 nm, SPE LAZMA, Russia) was installed on the dorsal surface of the animal's tail (in the middle of the tail length) with a minimum gap. The skin area where the probe tip was installed was marked with a permanent marker for subsequent measurements in the same place. Cutaneous blood flow was measured for 5 minutes. The mean value of skin perfusion (M, AU) was analyzed.
In this study, we used a modified occlusion test to record post-occlusive reactive hyperemia (PORH) in rats using LDF. This method has been used for many years in clinical functional diagnostics to assess the reserves of microcirculation and vascular reactivity [15]. Sphygmomanometer cuff, which is a component of a system for non-invasive pressure measurement in rodents (Systola, Neurobotics LLC, Russia), was applied to the proximal tail of the animal. The cuff was connected to a pressure gauge and a rubber bulb. After registration of cutaneous blood flow at baseline, the cuff was inflated to a pressure of 200-240 mm Hg and kept at this level for 3 minutes. After rapid cuff deflation, blood flow was recorded for the next 5 minutes. The following indicators of PORH in the skin of the tail were analyzed: maximum blood flow after removal of occlusion (Mmax, AU); blood flow reserve (BFR= Mmax /M x 100 %); cutaneous vascular conductance (CVC= Mmax /MAP) [16].
Gases and acid-base state (ABS) of arterial blood (рО2, рСО2, рН, HCO3, ВЕ, SaO2, lactate) were determined using CG4+ cartridges for the iSTAT 1 analyzer (Abbott Point of Care Inc., USA). The volume of the blood sample was 0.2 ml.
Venous blood samples were collected to determine the serum concentrations of biomarkers of brain damage: S100 calcium binding protein B (S100b) and neuron-specific enolase (NSE) by enzyme-linked immunosorbent assays (Cloud-Clone Corp., USA). The volume of the blood sample was 1.0 ml for each biomarker.
HR, MAP, M, gases and ABS of arterial blood were measured at baseline (BL), 55 minutes after hemorrhage (H55), 5 minutes after reperfusion (R5), and 24 hours (R24h) after reperfusion. PORH in the skin of the tail of a rat was evaluated at baseline (BL) and 24 hours after reperfusion (R24h).
For histological examination the internal organs were fixed in 10% buffered formalin for 48 hours. Tissue samples were processed, embedded in paraffin. Sections 6 μm thick were stained with hematoxylin and eosin (H&E) (lung, kidney, and heart) or cresyl violet (the brain). The quantitative analysis of highly sensitive to hypoxia neuronal populations of the hippocampus was performed. For this purpose on the images of hippocampal sections obtained with the help of light microscope Nikon Eclips Ni light microscope and digital camera at ×40, using NIS-Elements BR software (Nikon Corp., Japan) the number of unchanged pyramidal neurons in hippocampal fields CA1 and CA3-4 of the left hemisphere were counted manually in blind design conditions. Neurons with a clearly defined ellipsoid or round nucleus and a clearly visible nucleolus located in the nucleus center were considered unchanged, still viable. The figures obtained were reduced to 1 mm of the of cell layer length.
To assess neuronal damage in the core, immunohistochemical study of a neuronal nuclei marker (NeuN), was performed with the use of primary polyclonal antibodies (NeuN, dilution 1:200, Invitrogen, USA) and visualisation system Dako REAL EnVision Detection System, Peroxidase/DAB+ (DAKO, Denmark).
Statistical data analysis was performed using the Excel and Statistica 13.0 software package. The significance of differences in variables between groups was assessed using the Mann–Whitney U test. Differences were considered significant at p<0.05. Analyzed values are presented as Me (LQ; HQ).
During the experiments, four animals (two in the HS+AB group, one in the HS+BES group and one in the sham group) had complications of anesthesia and vascular catheterization (respiratory depression, unintentional bleeding, circulatory arrest upon induction of blood loss). These animals were excluded from the study and were not included in the analysis. In groups HS+AB and HS+BES, within 24 hours after hemorrhage and resuscitation, one animal in each group died (1 out of 5 and 1 out of 6, respectively).
At baseline, insignificant intergroup differences were revealed in the values of MAP, HR, BE, and arterial blood lactate. The groups did not differ in other parameters (Figures 1, 2 and 3). HR, MAP and M in the HS+AB and HS+BES groups were lower than in the sham group 55 minutes after hemorrhage (Figure 1). In the gases and ABS of arterial blood during this period compensated metabolic acidosis was detected in groups HS+AB and HS+BES (pH was within the normal range, a decrease in pCO2, HCO3- and BE, an increase in lactate levels), which was not observed in the sham group (Figure 2). Five minutes after fluid resuscitation, the HR in the HS+BES group was higher and MAP lower than in the HS+AB and sham groups. M did not differ between groups during this period (Figure 1).
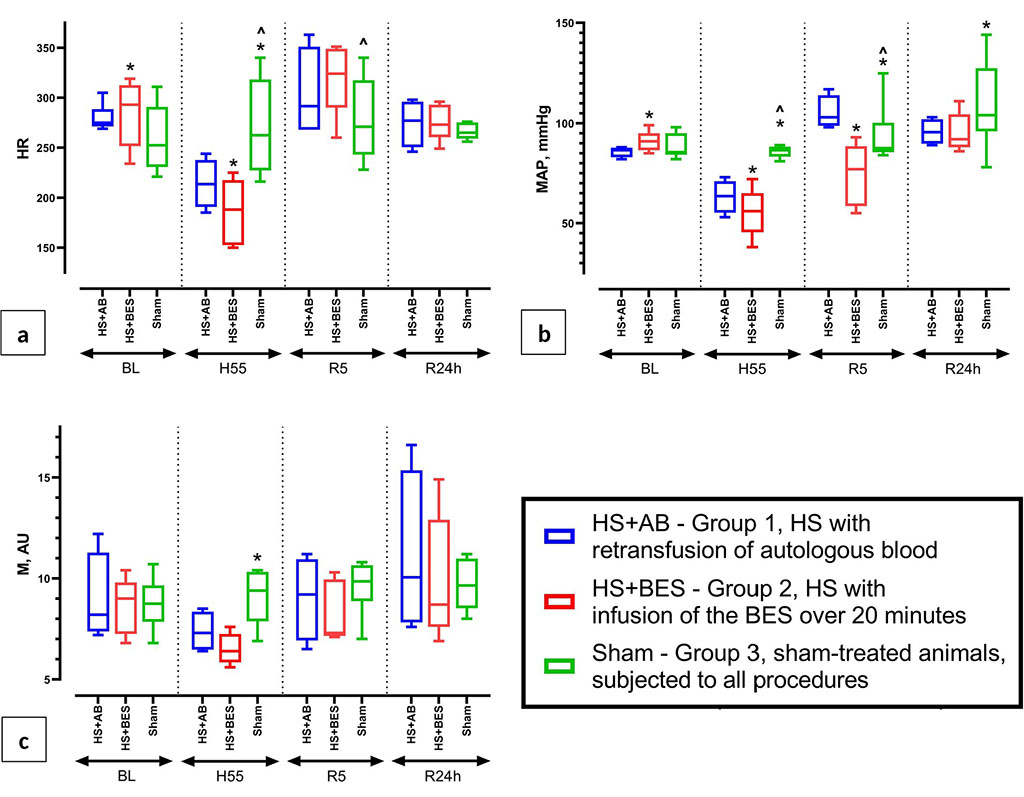
Figure
1 (a, b, c) Dynamics of heart rate (HR), blood pressure (MAP) and
mean value of skin perfusion (M) at baseline (BL), 55 minutes after
blood loss (H55), 5 minutes and 24 hours after reperfusion (R5 and
R24h).
Note:
* - p<0.05 vs HS+AB (group 1); ^ - p<0.05 vs HS+BES (group 2)
The groups did not differ in HR and M 24 hours after the resuscitation. MAP in groups HS+AB and HS+BES was moderately reduced compared to the sham group (Figure 1). In gases and ABS of arterial blood during this period in the HS+AB group, compared with the sham group, pCO2 was lower and pO2 was higher, while no differences were found in these parameters with the HS+BES group. In group HS+AB lactate levels were lower than in the HS+BES and sham groups (Figure 2).
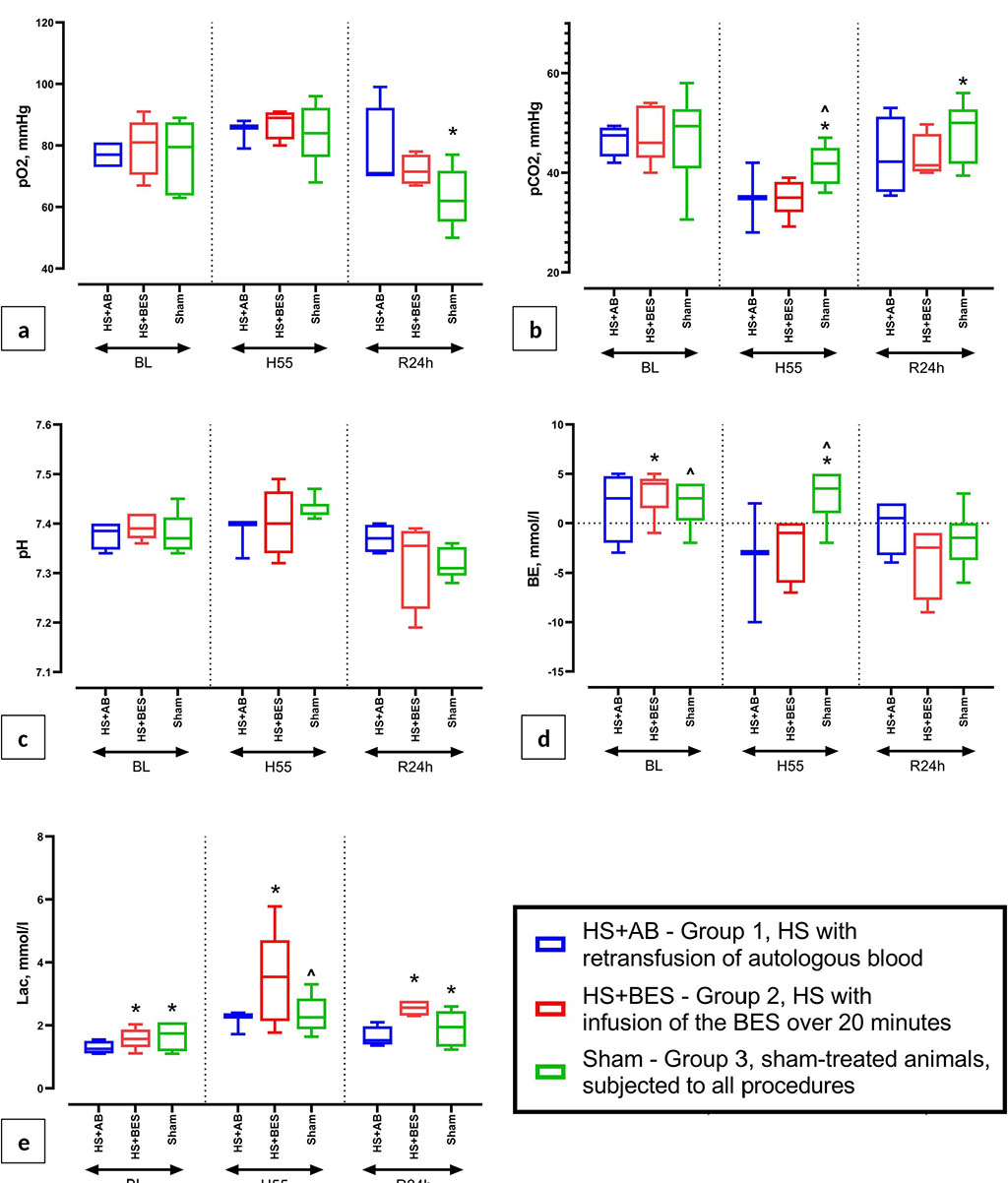
Figure
2 (a, b, c, d, e) Dynamics of arterial blood gases and ABS (pO2,
pCO2, pH, BE, lactate) at baseline (BL), 55 minutes after blood loss
(H55) and 24 hours after HS and reperfusion (R24h).
Note:
* - p<0.05 vs HS+AB (group 1); ^ - p<0.05 vs HS+BES (group 2).
Animals did not differ in the parameters of PORH (Mmax, BFR and CVC) in the skin of the tail both at the baseline and 24 hours after hemorrhage and resuscitation (Figure 3).
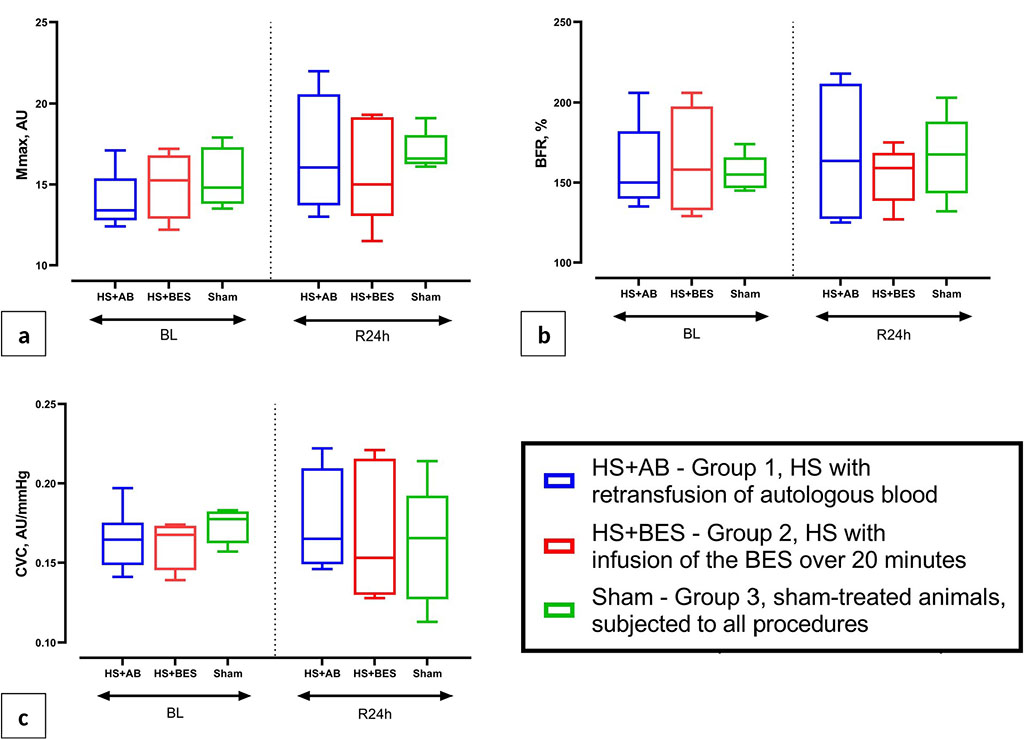
Figure 3 (a, b, c) Indicators of an occlusive test (PORH) on the rat tail: maximum blood flow after removal of occlusion (Mmax, AU), blood flow reserve (BFR, %) and the index of cutaneous vascular conductance (CVC) at baseline (BL) and 24 hours after HS and reperfusion (R24h).
Analysis of serum concentrations of biomarkers of brain damage 24 hours after the hemorrhage and resuscitation showed that in the HS+BES group the level of S100b protein was lower than in the HS+AB and sham groups. There were no intergroup differences in NSE levels (Figure 4).
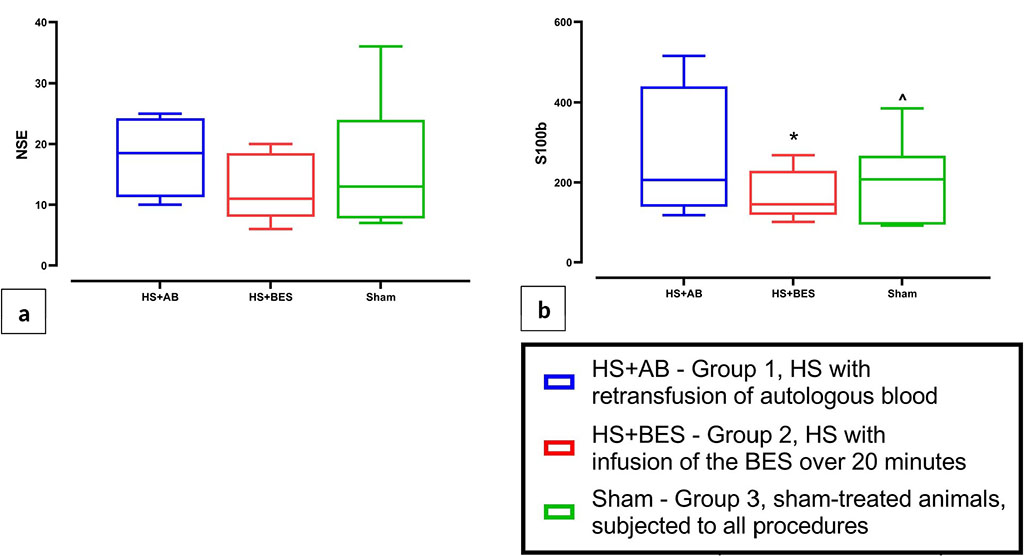
Figure
4 (a, b) Serum concentrations of brain damage biomarkers (NSE and
S100b) 24 hours after HS and reperfusion.
Note:
* - p<0.05 vs HS+AB (group 1); ^ - p<0.05 vs HS+BES (group 2)
Histological examination of the internal organs revealed morphological signs of circulatory disorders of varying severity in the HS+AB and HS+BES groups. In the lungs, a mosaic pattern of tissue damage was determined. In most cases, there were areas of atelectasis (Figure 5a, c), intraalveolar edema (Figure 5c), emphysema (Figure 5b). Atelectasis and distelectasis alternated with areas of emphysema and thinning of the interalveolar septa. There were hemorrhages in the alveoli and interalveolar septa (Figure 5а, c), as well as cell infiltration into the perivascular space (Figure 5a). The revealed changes were typical for both HS+AB and HS+BES groups.
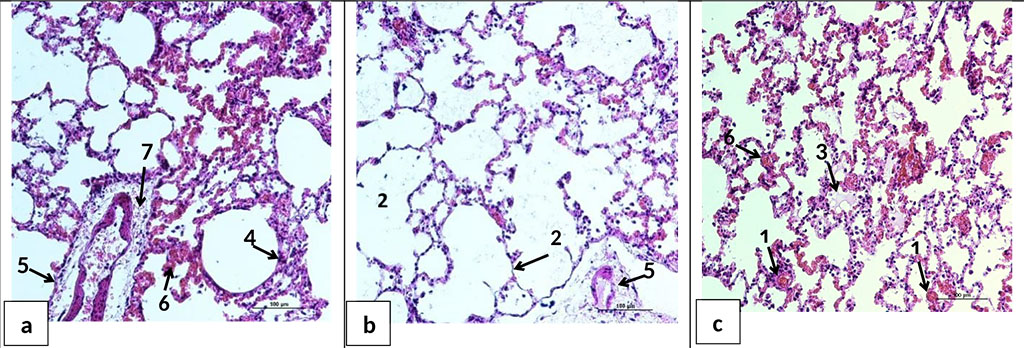
Figure 5. Microphotographs of lung sections 24 hours after HS and reperfusion. a, b, c - parts of the lungs of animals of HS+BES group. Full blood pulmonary vessels (1); thinning of interalveolar septa, emphysema (2); edematous fluid in the alveoli (3), dystelectas (4), perivascular edema (5), hemorrhages in the alveoli and interalveolar septa (6), inflammatory/leukocyte cell infiltration into the perivascular space of vessels (7). H&E staining. Ob. ×20.
In the kidneys, heterogeneous zonal blood filling of microvessels and signs of protein hydropic dystrophy of the tubular epithelium were observed. In group HS+AB, there was a slight swelling of the renal tubules and glomeruli, as well as swelling of the walls of the cortical arteries (Figure 6a). Animals of group HS+BES had a pronounced edema of epithelial cells of the renal tubules and edema of the glomerular capsule. In addition, in three out of five animals of HS+BES group, foci of necrosis of the glomeruli and tubules of the cortical region were noted (Figure 6b). Necrosis of the epithelium of the collecting ducts of the medulla was also observed (Figure 6c).
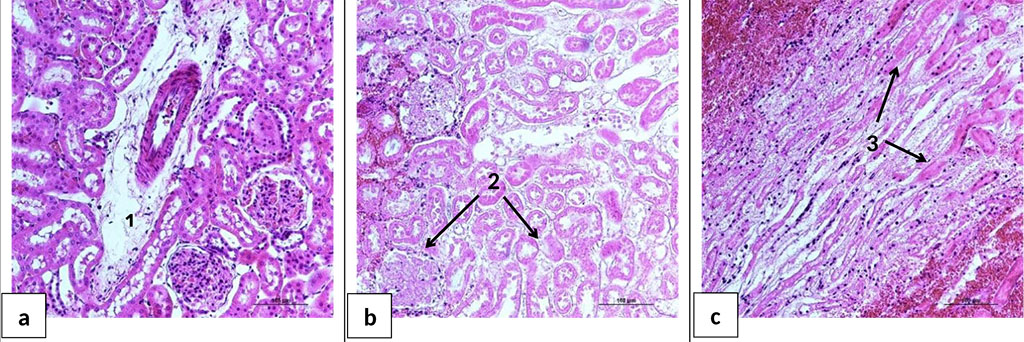
Figure 6. Microphotographs of kidney sections 24 hours after HS and reperfusion. a - the cortical layer of the kidney of the rat of the group HS+AB. b - the cortical layer of the kidney of the rat of the group HS+BES. c - the medulla of the kidney of the rat of the group HS+BES. 1 - perivascular edema; 2 - necrosis of the renal tubules and glomeruli of the cortical layer; 3 - necrosis of the epithelium of the collecting ducts of the medulla. H&E staining. Ob. ×20.
In the heart, fragmentation and wave-like deformation of muscle fibers were determined (Figure 7a, b), which indicates an impaired contractility of the heart. Heterogeneous blood filling, capillary dilatation, perivascular edema, interstitial tissue edema, small diapedetic hemorrhages, and intravascular sludge were also noted (Figure 7c). The revealed changes were typical for both group HS+AB and HS+BES.
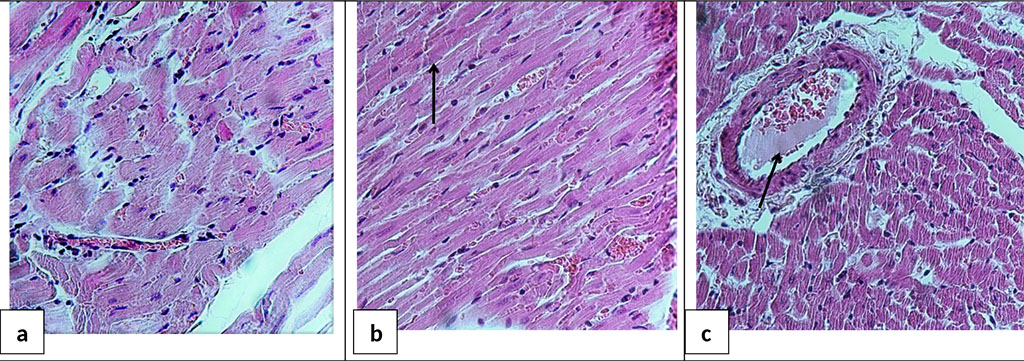
Figure 7. Microphotograph of the heart sections 24 hours after acute HS and reperfusion. a - area of compression and compaction of muscle fibers; b - fragmentation of Z-disks (marked with an arrow); c - sludge in a vessel (marked with an arrow). H&E staining. Ob. ×40
The study of brain sections stained for the NeuN protein, a specific marker of nerve cells, sporadic small foci of altered (ischemic) pyramidal neurons in the sensorimotor cortex were detected in HS+BES group (Figure 8b). In group HS+AB, there were no histological signs of brain damage (Figure 8a). Quantitative analysis of neuronal populations of the hippocampus did not reveal differences between the groups in the density of unchanged neurons in the pyramidal layer of fields CA1 and CA3-4 (Figure 8c).
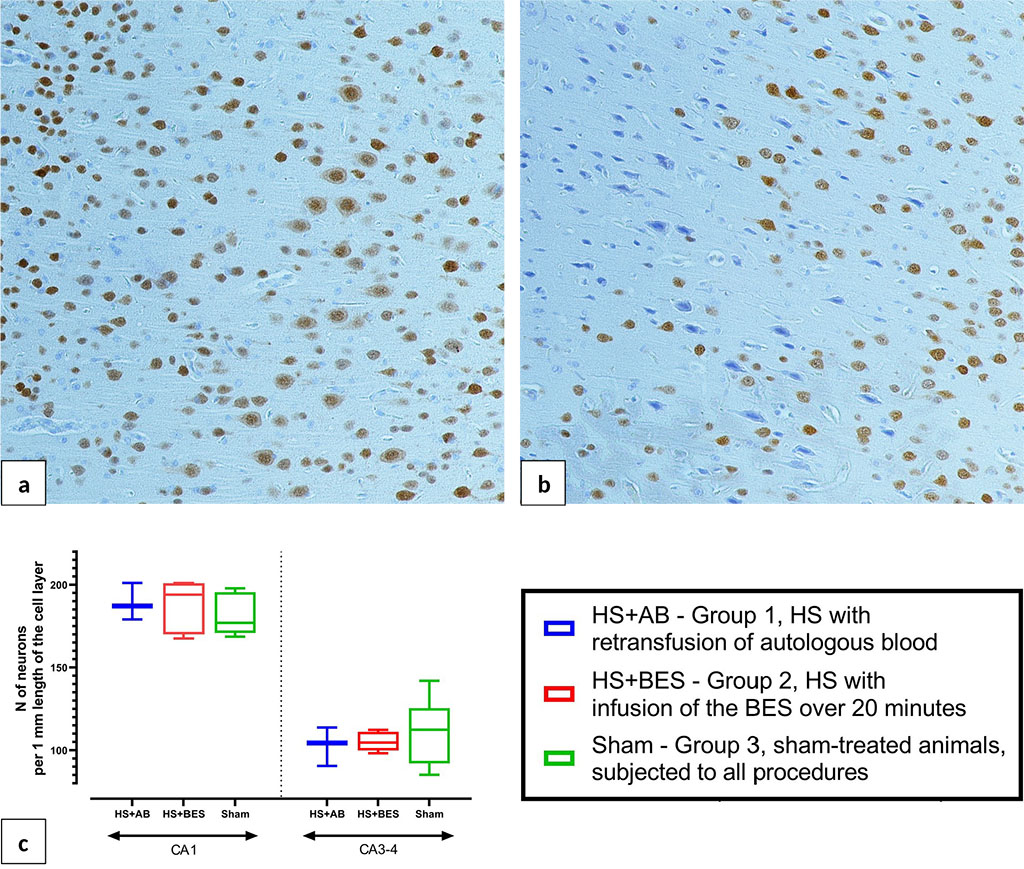
Figure 8. Microphotographs of the brain frontal sections 24 hours after HS and reperfusion. a - normal structure of the cerebral cortex. b - focus of ischemia in the cerebral cortex of HS+BES rats, immunohistochemical staining for the NeuN protein, peroxidase-antiperoxidase method, coverstaining with hematoxylin, ob. ×20. c - density of viable unchanged pyramidal neurons in fields CA1 and CA3-4 of the hippocampus.
In this work, changes in the hemodynamic and metabolic parameters were studied in an animal model of HS (class III of four [17]), followed by fluid resuscitation with either BES (HS+BES group) or a combination of the retransfusion of autologous blood with the same BES (HS+AB group). The groups did not differ in the level of 24-hour mortality (one animal in each group), however, the exclusion of four more animals from the study led to a high variability of the studied parameters, which explains the revealed intergroup differences in several variables already at baseline.
The standard rapid fixed-volume hemorrhage model
used in our work allows to study the natural dynamics of the
pathological process, and also has similarities with the development
of HS in patients with arterial bleeding. Blood loss naturally led to
the development of arterial hypotension, peripheral hypoperfusion
(decreased M values) and metabolic lactic acidosis in groups HS+AB
and HS+BES. A feature of this series of experiments was the
development of relative bradycardia in the experimental animals in
the posthemorrhagic period, which can be explained both by the effect
of combined anesthesia without the use of atropine and by the high
rate of hemorrhage (35% of the EBV for 5 min). The animals breathed
spontaneously; therefore, the compensated nature of acidosis after
blood loss was explained by the development of pulmonary
hyperventilation (decrease in arterial pCO2).
Retransfusion of autologous blood in combination with Sterofundin
isotonic (HS+AB group) restored MAP 5 minutes after resuscitation
better than an isolated infusion of the same crystalloid in a triple
volume of blood loss (HS+BES group). Nevertheless, 24 hours after HS
and fluid resuscitation in animals of both groups, blood pressure,
cutaneous microcirculation and arterial blood ABS were restored, but
signs of hyperventilation and a moderate decrease in blood pressure
(MAP 90-100 mm Hg) persisted.
The levels of serum biomarkers of brain damage 24 hours after HS and reperfusion turned out to be contradictory. While NSE levels did not differ between the three groups, S100b protein levels were lowest in the HS+BES group. This fact, however, may be explained by hemodilution (S100b values are lower than in the sham group) and the high variability of the values of this biomarker in rats. Therefore, the studied relatively small sample of animals may not be sufficient for the correct interpretation of the obtained values of the S100b biomarker. Quantitative analysis of neuronal populations in fields CA1 and CA3-4 of the hippocampus 24 hours after HS and fluid resuscitation showed no differences between groups in the density of viable pyramidal neurons. Nevertheless, in the HS+BES group, some animals had small foci of ischemic brain injury, as well as areas of necrosis in the kidney (evidence of acute kidney injury). These data indicate that, despite the effective correction of a number of hemodynamic and metabolic parameters, fluid resuscitation with only a BES (a balanced crystalloid) is not so effective in preventing the development of ischemic injuries of internal organs in HS. It should be noted that in another study, the use of Sterofundin isotonic for fluid resuscitation in a rat model of HS contributed to a decrease in the severity of damage to internal organs after HS [5].
In general, the results obtained are in good agreement with the data of previous studies. There is already strong evidence to support the use of BESs for the correction of hypovolemia in patients with HS and other critical conditions, since infusion of large volumes of saline has a number of serious side effects: hyperchloremic metabolic acidosis, damage to the vascular endothelium with increased microvascular permeability, acute kidney injury and other manifestations of organ dysfunction [9, 11, 18]. It has also been shown that lactated Ringer’s and other crystalloids containing lactate as a buffer base are less effective in correcting water-electrolyte and metabolic disorders in HS compared to solutions containing bicarbonate, acetate or malate [12, 15, 19]. Against this background, Sterofundin isotonic and similar BESs containing acetate or malate can be considered as crystalloids of choice in the complex intensive care of HS [20].
At the same time, a meta-analysis of preclinical studies has shown that fluid resuscitation for HS with only crystalloids (including balanced crystalloids) is associated with higher levels of arterial lactate compared with colloids and blood components [21]. Therefore, despite the obvious fact that a full-fledged treatment of severe blood loss with crystalloids alone is impossible, our experimental study suggests the fundamental possibility of compensating for circulation, respiration, and the metabolic status of the body 24 hours after class III hemorrhage without resorting to transfusion of donor blood.
One of the objectives of this study was to test a new (for preclinical studies) non-invasive method for assessing microcirculation, namely, an occlusive test on the tail of a rat to register PORH. The occlusion test in combination with LDF and other methods of recording peripheral blood flow (ultrasound, photoplethysmography, etc.) has been used in clinical functional diagnostics and scientific research to assess blood flow reserve, vascular reactivity, and a number of other functional parameters of regional blood circulation [15, 22]. In experimental studies, attempts have already been made to adapt the occlusion test for rodents in order to assess vascular reactivity and microcirculation reserve in pathological conditions [23]. However, these options have such disadvantages as the invasiveness of the technique in the study of muscle blood flow; the need for anesthesia (with the location of the cuff on the thigh) and unreliable contact of the optical probe with the tissue surface under study. The modification of the rat tail occlusion test used in this study does not have these disadvantages and can be widely used in the practice of preclinical studies for a more detailed assessment of microcirculation, including in non-anesthetized animals.
This study did not reveal intergroup differences in variables of PORH in the skin 24 hours after HS and fluid resuscitation. This, on the one hand, is in good agreement with the dynamics of other functional parameters studied in this series of experiments, and on the other hand, indicates the need for further validation of a new technique for assessing microcirculation in critical conditions and under different types of anesthesia.
Both heparinized autologous blood and a balanced electrolyte solution containing acetate and malate equally restore blood pressure, skin microcirculation, and arterial blood acid-base balance 24 hours after class III hemorrhage. However, fluid resuscitation of hemorrhagic shock with only a balanced electrolyte solution (without transfusion of blood components) is associated with a higher level of arterial blood lactate, as well as with potential ischemic/reperfusion injury of the brain, lungs, and kidneys.