- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 3: e1. DOI 10.35630/2199-885X/2022/12/3.6
There are a number of reasons that lead to inadequate salivation or complete cessation of salivation. Among others, one of the main can be systemic diseases like Sjögren’s syndrome, and the use of medication, dysbiotic changes in oral cavity and stress. Most often, patients with xerostomia disturbed itching and burning in the mouth, taste disturbance, difficulty in speaking and swallowing food (especially dry), and difficulty wearing dentures. Hypofunction of salivary glands can lead to different outcomes in the mouth. The most common are, first of all, the loss of gloss of the oral mucosa, it atrophic changes, the appearance of fissures and lobes on the back of the tongue, the development of angular cheilitis, dysbiotic change, and the development of multiple caries. If xerostomia oral mucosa requires compliance with the rules of oral hygiene and constant daily moisturizing. The best method of relief of patients is to stimulate the secretion and the mandatory use of wetting agents to the oral mucosa. A thorough intra-oral and extra-oral clinical examination is important for diagnosis. Treatment may include the use of salivary substitutes (Biotene), salivary stimulants such as pilocarpine, ongoing dental care, caries prevention, a review of the current prescription drug regimen and possible elimination of drugs having anticholinergic effects. The article presents the results of histological and immunohistochemical features of the structure of the main excretory duct of the parotid salivary gland for the presence of muscle structures in it and clarifying the possibility of their influence on the development of xerostomia.
Keywords: dry mouth, inadequate salivation, parotid salivary gland, immunohistochemical analysis.
Dysfunction of the large salivary glands, manifested in the form of xerostomia, is currently a common problem in patients seeking dental care [5,7,14]. Among the causes of the development of xerostomia are autoimmune, endocrine diseases, side effects of medications, neuropressive states, dysbiosis, the effects of radiation therapy and other pathology of the salivary glands [3,13,17]. Separately, we can single out a group of patients with a symptom of dryness of the oral cavity, who have no history of the above pathological changes or abnormalities in the structure of the large salivary glands [6,16]. The problems of the etiology of this type of xerostomia and its treatment methods are currently relevant.
The structure of the excretory ducts of the large salivary glands has been studied for a long time. It is well known that the excretory ducts of the parotid salivary gland are a system of insertion, striated, intra-lobular and interlobular ducts, which, as a rule, are combined into one main parotid duct. The walls of the parotid duct along its length are lined with multilayer cubic epithelium, in the area of the mouth with multilayer flat epithelium [2,8,15].
Erichev I.V. in his work indicates the presence of reservoir and valve structures in the duct system of large salivary glands, which together with muscle elements participate in the transport of saliva. A number of sources also describe the presence of a muscular component of the main excretory duct of the parotid salivary gland, represented by a system of reservoir and valve structures, the action of which provides unidirectional saliva flow [1,10,12]. Thus, the spasm of these muscle structures can lead to a violation of the function of the parotid salivary gland and, accordingly, xerostomia [4,9,11]. The above causes the relevance of clinical, morphological and immunohistochemical studies of the walls of the main excretory duct to identify components of muscle structures in them and clarify the possibility of their influence on the development of xerostomia.
Aim. To study the histological and immunohistochemical features of the structure of the wall of the main excretory duct of the parotid salivary gland for the presence of muscle structures in it and to clarify the possibility of their influence on the development of xerostomia.
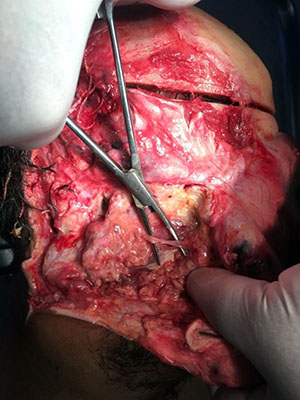
The study was conducted at the Departments of Surgical Dentistry, Pathological Anatomy, Operative Surgery and Topographic Anatomy of the Moscow State Medical University named after A.I. Evdokimov (Moscow, Russia). The main excretory ducts of 10 parotid salivary glands were completely isolated and excised during autopsies (Fig. 1, 2). The criteria for choosing fragments were the absence of any diseases, injuries and pathological processes in the maxillofacial region and the salivary glands. The age of men (4 cases) and women (6 cases) who died from acute and chronic forms of coronary heart disease ranged from 39 to 88 years.
From each main excretory duct of the CSF from its proximal to distal sections, with a step of 0.3 - 0.5 cm, 6 – 9 samples were cut out, transversely to the axis of the duct, fixed in 10% neutral buffered formalin and, according to the generally accepted method, poured into paraffin blocks. Histological sections, 3 microns thick, were made using the NM355S rotary microtome (Thermo Scientific, Germany) from the obtained paraffin blocks (samples in paraffin blocks were oriented transversely to the duct axis) on the Leica microtome (Germany), stained with hematoxylin and eosin and used for immunohistochemical reactions.
For immunohistochemical examination, histological sections were placed on special slides coated with adhesive. Endogenous peroxidase in dewaxed sections was blocked with 3% hydrogen peroxide. Monoclonal antibodies to alpha-smooth muscle actin (α-SMA – mouse monoclonal antibodies, clone 1A4), HHF35 (monoclonal mouse antibodies to muscle actin, clone HHF35), cytokeratin-7 (CK-7– mouse monoclonal antibodies, clone OV-TL 12/30) and desmin (mouse monoclonal antibodies, clone D33). In this study, antibodies produced by Cell Marque, USA, were used in a ready-to-use dilution. The study of the preparations and microphotography were carried out using a Leica DM LB microscope (Germany).
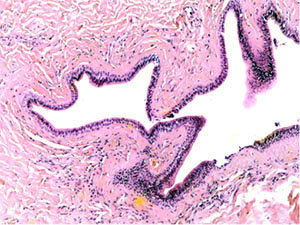
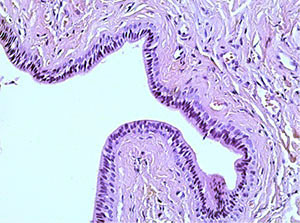
A study of histological preparations of the main excretory duct of the CSF stained with hematoxylin and eosin (Fig. 3, a, b – ×60, c, d - ×250) showed that it is lined almost to the mouth with a prismatic two-layer epithelium, where it passes into a multilayer squamous epithelium. Externally, it is surrounded by connective tissue, represented mainly by concentric oriented bundles of collagen fibers with single fibrocytes and fibroblasts. In the subepithelial layer under the basement membrane of the epithelium, a denser arrangement of bundles of collagen fibers is determined. Vessels of different calibers are located in the periductal connective tissue and the surrounding adipose tissue. Muscle cells in histological preparations of the walls of the main excretory duct of the CSF were not visualized.
According to the results of an immunohistochemical study of the main excretory duct with monoclonal antibodies to alpha-smooth muscle actin, pronounced expression of α-SMA (alpha-smooth muscle actin) was demonstrated in the cells of the walls of various vessels (Fig. 4). At the same time, there is a lack of expression in the cells of the wall of the main excretory duct (Fig. 5).
When conducting an immunohistochemical study of the main excretory duct with monoclonal antibodies to mouse actin (clone HHF35), a similar pattern was found: pronounced expression of HHF35 in the cells of the walls of various vessels and the absence of expression in the cells of the wall of the main excretory duct, indicating the absence of cells with muscle differentiation in it (Fig. 6, 7).
The results of immunohistochemical studies of the main excretory duct of the CSF with desmin have demonstrated the absence of expression in the duct wall (Fig. 8).
Immunohistochemical examination of the main excretory duct with monoclonal antibodies to cytokeratin-7 revealed pronounced expression in the cells lining its walls from the inside, which indicates in favor of their epithelial nature and the absence of expression in other cells (Fig. 9, 10).
 |
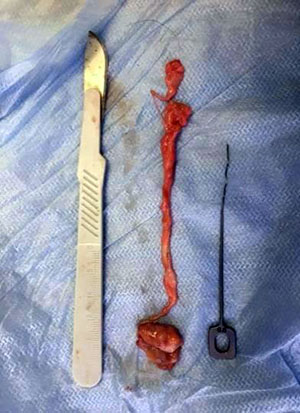 |
Fig. 1 Selected fragment of the parotid duct |
Fig. 2 The removed fragment of the parotid duct |
 |
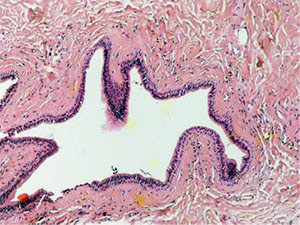 |
Fig. 3a The excretory duct of the salivary gland. Staining with hematoxylin and eosin – x 60 Fig. |
3b The excretory duct of the salivary gland. Staining with hematoxylin and eosin – x 60 |
 |
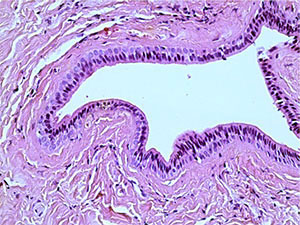 |
Fig. 3c into the excretory duct of the salivary gland. Staining with hematoxylin and eosin – x 250 fig. |
3d Excretory duct of the salivary gland. Staining with hematoxylin and eosin – x 250 |
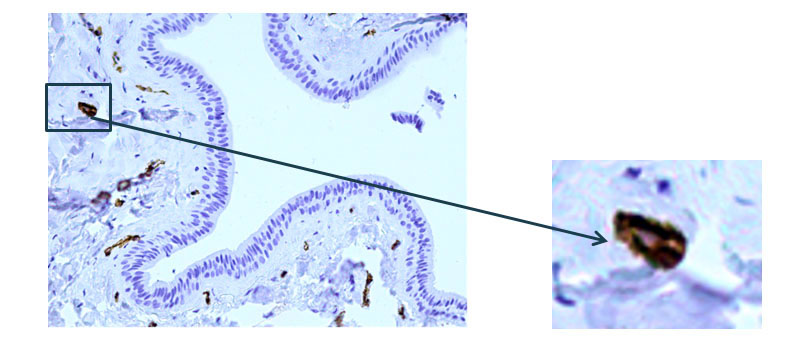
Fig. 4 The excretory duct of the salivary gland. Indirect immunoperoxidase method with antibodies to α-SMA, x 200.Pronounced expression of α-SMA (alpha-smooth muscle actin) in the cells of the walls of various vessels.
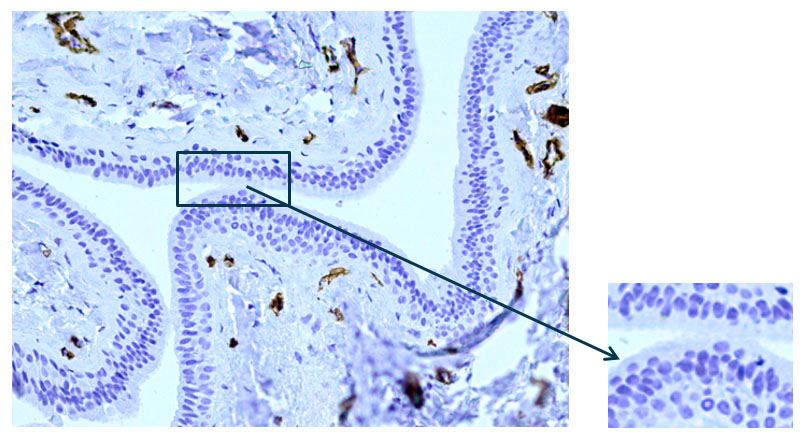
Fig. 5 The excretory duct of the salivary gland. Indirect immunoperoxidase method with antibodies to α-SMA, x 200.Lack of expression in the cells of the excretory duct wall.
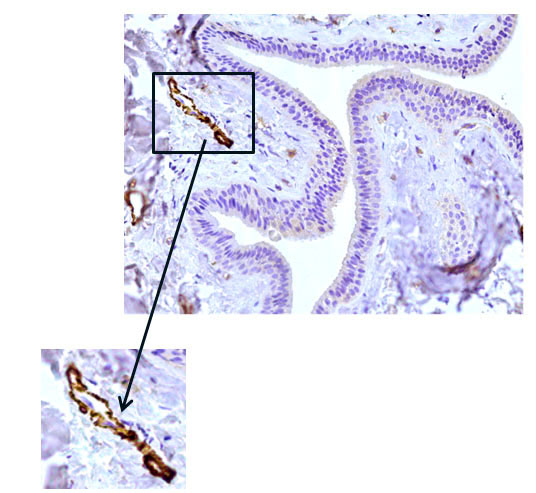
Fig. 6 The excretory duct of the salivary gland. Indirect immunoperoxidase method with antibodies to HHF35, x 200. Pronounced expression of HHF35 in the cells of the walls of various vessels.
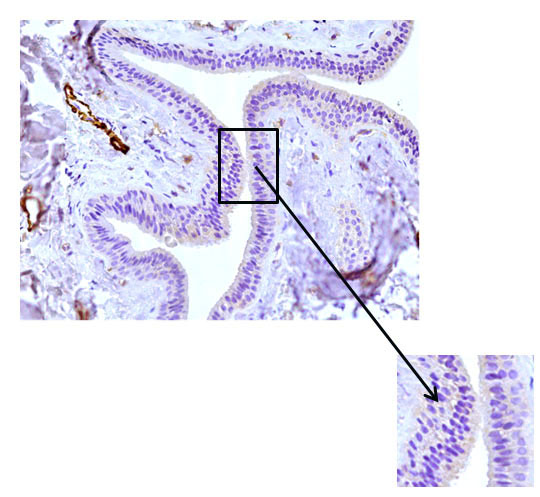
Fig. 7 The excretory duct of the salivary gland. Indirect immunoperoxidase method with antibodies to HHF35, x 200. Lack of expression in the cells of the excretory duct wall.
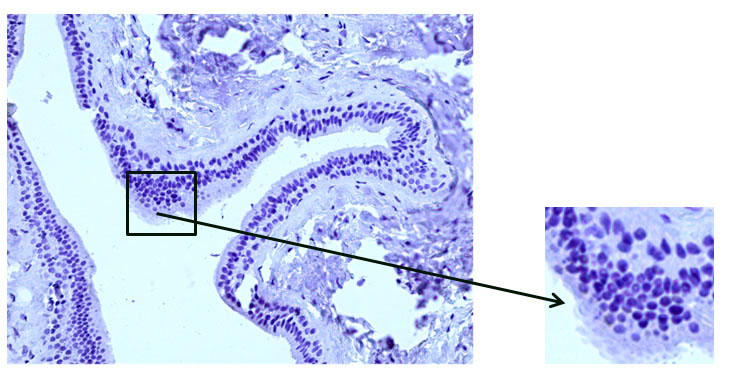
Fig. 8 The excretory duct of the salivary gland. Indirect immunoperoxidase method with antibodies to desmin, x 200. Absence of desmin expression in duct wall cells.
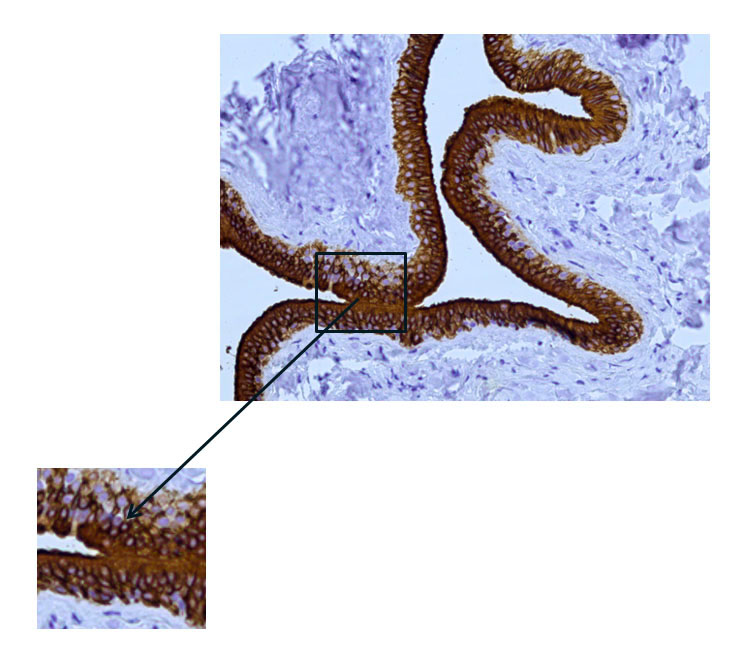
Fig. 9 The excretory duct of the salivary gland. Indirect immunoperoxidase method with antibodies to CK7, x 200. Pronounced expression of cytokeratin 7 (CK7) in the duct epithelium.
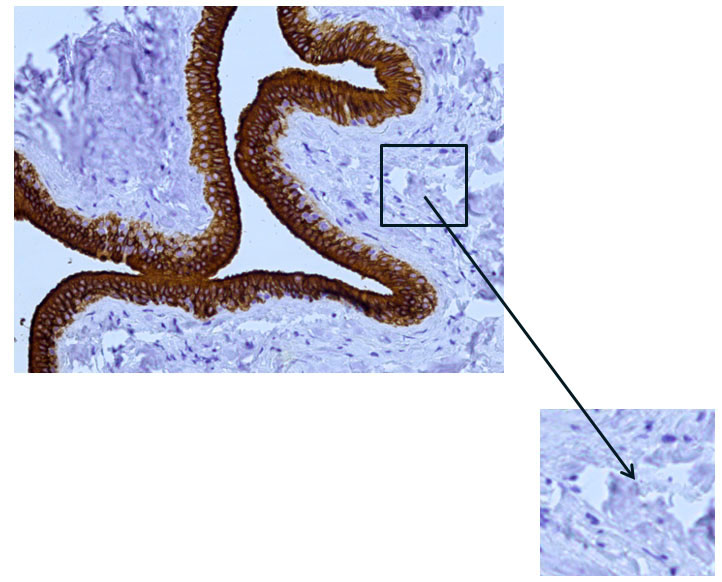
Fig. 10 The excretory duct of the salivary gland. Indirect immunoperoxidase method with antibodies to CK7, x 200. Absence of cytokeratin 7 (CK7) expression in other cells.
Thus, the results of the histological examination of the walls of the main excretory duct of the CSF throughout its entire length did not reveal the elements of the muscular nature. In turn, this is confirmed by a negative immunohistochemical reaction with specific markers of muscle structures, which demonstrates the absence of cells with muscle differentiation in the wall of the main excretory duct of the CSF.