- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
DOI 10.35630/2199-885X/2022/12/3.3
Visual-spatial perception in children and adolescents is largely determined by age-specific features of the structure and hemodynamics in the associative areas of the cerebral cortex.
Aim. The aim of the study was to investigate the quantitative ratios of vascular and glial components in the oculomotor area 8 of the prefrontal cortex and the 37aс subarea of the posterior associative cortex involved in visual-spatial analysis.
Methods. We studied 27 left large hemispheres of the brains in male subjects aged 10 to 17 years, grouped in annual intervals. We studied specific volumes of intracortical blood vessels and gliocytes in the external pyramidal layer on paraffin-embedded cortical slices impregnated with silver nitrate according to Peters with Nissl dyeing using a stereometric method.
Results. We found that microstructural changes in the adolescent neocortex resulted in a decrease in the relative content of intracortical microvessels and an increase in the specific volume of gliocytes. The structural changes occur heterochronously: in the oculomotor area 8 of the prefrontal cortex, at 11-12 years of age, and in the subarea 37aс, at 12-13 years of age.
Conclusion. The data obtained testify to a significant morpho-functional restructuring of trophic support and metabolic processes in the associative areas of the neocortex controlling visual-spatial perception.
Keywords: male children and adolescents, neocortex, gliocytes, intracortical blood vessels, stereometry
The function of visual-spatial perception is controlled by various cortical centers, including those located in the temporoparieto-occipital subregion and the frontal cortex. Its violation, in addition to deficit of attention, working memory and the ability to orient in space, can lead to dyslexia, dysgraphia and other abnormalities in the implementation of cognitive functions [8, 15]. The cerebral organization of visual-spatial perception improves over a long period of time during postnatal ontogenesis. Adolescents in the initial stages of puberty still experience certain difficulties in processing visual-spatial information that are not normally characteristic of adults [9]. Improvement of brain activity mechanisms is largely determined by age-related transformations in the structure and hemodynamics of the neocortex. Gliocytes and intracortical microvasculature play an important role in the metabolic maintenance of the functional activity of the cortex, which together with neuronal clusters form neurotrophic units in the neuro-glio-vascular ensembles of the cortex [12, 13].
Based on the above, the aim of the study was to examine the quantitative ratios of the vascular and glial components in the associative areas of the neocortex involved in visual-spatial perception in order to identify the features of their microstructural organization in children and adolescents.
Fragments of the large cerebral cortex from 27 left large hemispheres of male subjects aged 10 to 17, who died violently and without brain injury, were used as the material of the investigation. The Ethics Committee in the Institute of Developmental Physiology of the Russian Academy of Education allowed the collection of the necessary sectional material (protocol No. 3 of 23.05.1996) in forensic morgues in Moscow and the Moscow region. The cortical areas for the study were selected according to K. Brodmann's cytoarchitectonic map and the Atlas of the Cytoarchitectonics of the Human Cortex, edited by S.A. Sarkisov et al. (1955). Brain tissue fragments were excised in the anterior part of area 8 (frontal eye field, BA 8), and in subarea 37ac (SA 37ac) of the temporoparieto-occipital region, which is involved in determining the position of the visual object in space [7].
We grouped the studied material in annual intervals. Paraffin sections of 10 µm thickness were made in frontal projection and stained with Nissl cresyl violet after impregnation with silver nitrate according to Peters modification [2]. Digital images of preparations were analyzed using Image-Tools software (National Institutes of Health, USA) on a LOMO Biolam-15 microscope with a built-in USB camera UCMOS01300KPA (Altami, Russia). Volumetric ratios of the structural elements of the neocortex in different age groups were determined using the stereometric method [10] in its own modification. The relative specific volumes (SV) of intracortical blood vessels and gliocytes in cortical sublayer III3 were detected using a 4-node optical grid with a random step built into the program. A total of 850-1000 measurements of the studied structural components were taken at each age, achieving a reliability criterion of P=95%. In order to unify the quantitative data obtained from different slices, A. Abercrombie's formula [3] was used to calculate the number of microobjects taking into account the thickness of the slice. Significance of differences between mean group indices was determined by methods of variance statistics with calculation of the mean error and confidence interval with a significance level of P≥95% (p<0.05).
According to the data of stereometric analysis in children 10 years of age, the relative content of intracortical blood vessels was 14.3±1.0% and gliocytes 36.4±3.0% in the III3 sublayer of the prefrontal cortex in the BA 8. By 11 years of age, vascular SV significantly decreased 1.2-fold compared with 10 years of age and remained unchanged in adolescents (Fig. 1). By 17 years of age, the SV of intracortical vessels was 10.3±1.0%.
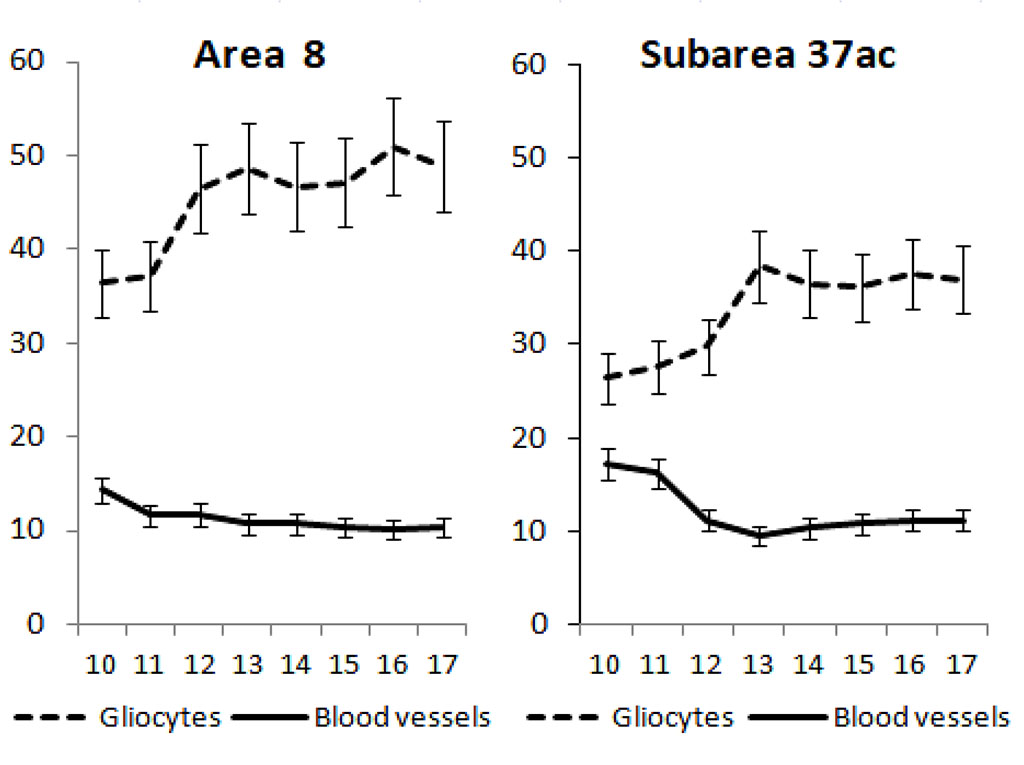
Fig. 1. Age-related changes in specific volumes of gliocytes and intracortical blood vessels in sublayer III3 of area 8 and subarea 37ac of the neocortex in boys aged 10 to 17 years. On the x-axis - age in years, on the y-axis - specific volumes in %, vertical bars - confidence interval at significance level p<0.05.
The relative content of glial cells increased 1.3-fold by the age of 12 compared to 10-year-olds and remained stable in adolescents from 13 to 16 years of age thereafter. By 17 years of age, the SV of gliocytes in BA 8 of the prefrontal cortex was 48.8±6.8%. In the external pyramidal layer of the posterior associative cortex in the SA 37ac in children 10 years of age, the SV of intracortical vessels and gliocytes were 17.2±2.4% and 26.4±2.4%, respectively, and did not change until age 11. By 12 years of age, the relative content of microvessels significantly decreased by a factor of 1.5 as compared to 10-year-old children.
Just as in the prefrontal cortex, in the posterior associative cortex in adolescents during the age interval from 13 to 16 years, the SV of intracortical blood vessels did not change and by 17 years was 11.2±2.8%. Gliocyte SV in children aged 11-12 remained stable. However, by age 13, it increased 1.3-fold compared to age 12 and remained unchanged later, amounting to 37.0±4.6% by age 17.
It follows from the obtained data that the change in the SV of intracortical vessels and the increase in gliocytes in boys at the prepubertal and early pubertal stages in the associative cortical areas followed a certain pattern. Initially, a decrease in vascular SV was detected, followed by an increase in the intracortical glial component over the next year, which probably had a compensatory-adaptive character.
Previously, it was shown in experimental models that under the influence of sex hormones in male rats there is a suppression of astrocytic glia functions, which may be a stimulus for a compensatory increase in its content in the cortex [5]. We believe that the phenomenon of decreased SV of intracortical vessels is the result of complex microstructural changes in the neocortex associated with a significant increase in the intracortical fibers in adolescents, as well as complication of the fibroarchitectonics, composition of neuronal clusters and neuroglio-vascular ensembles in general, which we showed earlier [11, 12].
We also assume, that decreased SV of intracortical microvessels is one of the causes of increased cerebral blood flow velocity in adolescents [1]. The increase in the content of astrocytic glia, which influence vascular tone, becomes understandable. The increase in gliocyte SV can occur due to hormonal activation of polydendrocytes, or NG2 cells in the external granular and pyramidal layers (II-III) of the cerebral cortex, which have high mitogenic properties, histogenetic potential to form different types of gliocytes in brain tissue, and are an active partner in neuron-glial interactions [14].
Age-related changes in the glial-vascular index in both studied areas were similar (Fig. 2), but, as can be seen from the figure, the increase in the glial component in the prefrontal cortex was more pronounced compared to the temporoparieto-occipital subregion.
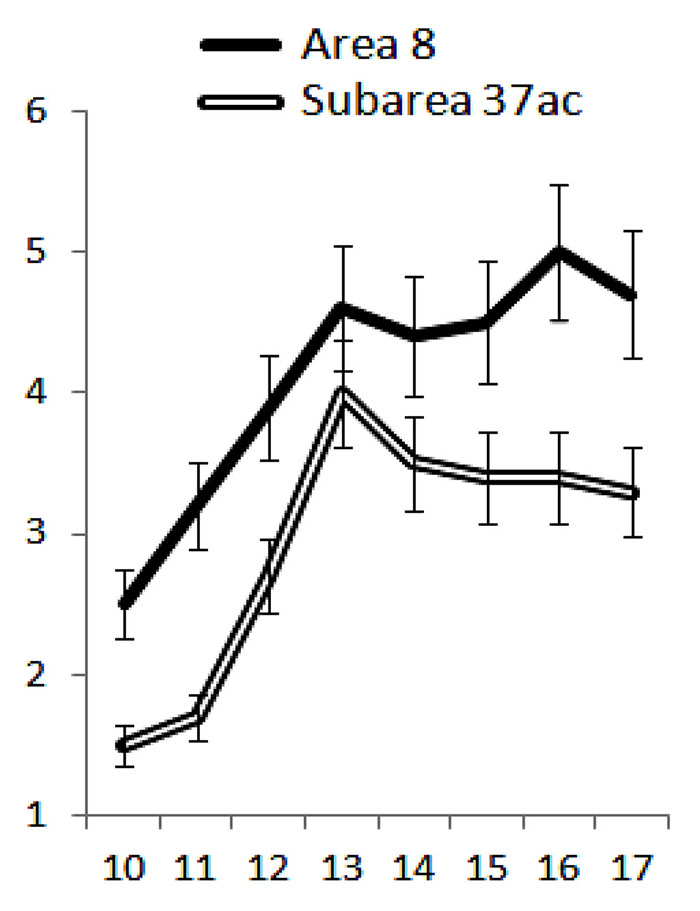
Fig. 2. Age-related changes in the gliovascular index (Igv) reflecting the ratio of gliocyte specific volumes to intracortical specific volumes in the associative zones of the neocortex of boys from 10 to 17 years of age. On the x-axis - age in years, on the y-axis - Igv in conventional units. Vertical bars are confidence interval at p<0.05.
It is generally accepted that in postnatal ontogenesis, the prefrontal cortex matures most late [6]. However, when comparing the timing of microstructural changes in the cortical areas involved in visual-spatial perception, we found that microstructural transformations in the frontal ocular field 8 were more intense and occurred 1 year earlier (at 11-12 years) compared with similar changes in the posterior associative cortex (at 12-13 years). The data obtained are consistent with the results of studies showing the outstripping development of perceptual visual-motor activity, in which the prefrontal cortex plays a fundamental role, in comparison with visual-spatial analysis, in which the temporoparieto-occipital subregion of the neocortex actively participates [4].
Microstructural changes in the external pyramidal layer of the associative areas of the neocortex in adolescents include a decrease in the relative content of intracortical microvessels and an increase in the specific volume of gliocytes. Structural transformations occur heterochronously: in the oculomotor area 8 of the prefrontal cortex - at 11-12 years old, and in the subarea 37ac - at 12-13 years old. The data obtained testify to a significant morpho-functional restructuring of trophic provision and metabolic processes in associative areas of the cerebral cortex controlling visual-spatial perception under conditions of tension of central regulatory systems with their participation in boys at the prepubertal and early pubertal stages of individual development.
The study was performed under the Strategic Academic Leadership Program in RUDN University «Priority-2030» of the Ministry of Education and Science; theme No. 030209-0-000.