- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 3: e1. DOI 10.35630/2199-885X/2022/12/3.26
The aim of the study was to assess the possibility of intraoperative decalcification of autografts from dentin using a solution of EDTA salts (ethylenediaminetetraacetic acid) 17% at an exposure of up to 10 minutes during alveolar bone plasty.
Materials and Methods: 19 patients aged 20 to 45 years, according to indications, 24 third molars were routinely removed, from which 48 plates 1.5-2 mm thick were made. The obtained samples were divided into 4 groups of 12 plates in each group. The samples of the first group were not treated with 17% EDTA salt solution, the samples of the 2nd group were treated for 1 minute, the 3rd group - 5 minutes, the 4th group - 10 minutes. After processing, all samples were washed under distilled water for 1 minute. Further, using a scanning electron microscope "FEI Quanta 250" (USA) in the area of enamel, dentin and dentin at the border with the pulp chamber of the prepared samples, the microstructure was investigated, and the qualitative and quantitative chemical composition of dental tissues was also studied.
Results: The amount of Ca in the studied samples of the 1st group: in the enamel area was - 42.32% ± 1.1; dentine – 42.25% ± 1.3; dentin at the border with the pulp chamber - 29.71% ± 1.5; group 2: in the enamel area - 48.95% ± 0.9.1; dentin – 37.39% ± 1.2; dentin at the border with the pulp chamber - 19.32% ± 1.1; group 3: in the enamel area - 39.3% ± 1.9; dentine - 38.3% ± 1.4; dentin at the border with the pulp chamber - 31.06% ± 1.1; 4th group: in the enamel area - 37.85% ± 0.8; dentine - 37.8% ± 1.5; dentine at the border with the pulp chamber - 26.6% ± 1.2 (p<0.05).
Conclusion: Thus, the treatment with 17% EDTA salt solution for up to 10 minutes exposure of dentin autografts used in the form of plates does not significantly affect their microstructure, qualitative and quantitative composition (CI = 95%), and the degree of demineralization. Hence, it is impractical to carry out this manipulation intraoperatively in alveolar bone plasty.
Keywords: EDTA, osteoplastic materials, bone grafting, autodentin, 3 molars, alveolar bone.
Alveolar bone and dentin of teeth have a common origin and similar chemical composition, which formed the basis for the use of dentin as an autograft in alveolar bone plasty [1,2,4].
Scientists demonstrate clinical cases of the use of autodentin as a graft in osteoplastic operations, both in the form of dentin crumbs and in the form of fragments of tooth roots, obtaining an increase in bone tissue up to 4.5 mm after 24 weeks [3,15,16].
According to a number of authors, one of the stages of bone grafting operations using autodentin is its preliminary demineralization. In a number of studies, it was found that a fully demineralized dentin matrix contributes to a reduction in the period of bone tissue formation compared to mineralized dentin [2,7].
To carry out the demineralization procedure, the authors of the studies used various acid solutions: HNO3, citric acid, HCl [2,4-8,10,11], the use of which is limited in clinical practice due to the lack of ready-made forms on sale and the high probability of chemical burns fabrics. As an alternative to obtain partially demineralized dentin grafts in the form of crumbs, the KometaBio Company (USA) proposed using a 10% EDTA solution [12], which is not available for open sale in Russia. In turn, solutions of sodium and potassium salts of EDTA are widely used in endodontic practice at concentrations from 15% to 17% to expand and pass root canals due to the ability to dissolve the mineral components of dentin and remove the smeared layer, but the opinions of researchers differ on these issues [13-15].
The ability to influence the structure of dentin grafts, leading to an acceleration of the formation of bone tissue by demineralization with EDTA salt solution, ambiguity of opinions about the effect of EDTA solution on the structure of tooth tissues and a limited number of clinical and laboratory studies of the use of this solution in the preparation of extracted teeth for the use of their tissues as autografts for bone reconstructive operations determined the purpose of our study.
Aim of study: to evaluate the possibility of intraoperative decalcification of autografts from dentin using a 17% EDTA salt solution for up to 10 minutes exposure during alveolar bone grafting.
For the study, 24 third molars were removed according to indications in a planned manner in 10 men (52.6%) and 9 women (47.4%) aged 20 to 45 years. From the extracted teeth, using a separation disc and cylindrical burs, 48 plates 1.5-2 mm thick were made under cooling with saline (Fig. 1), soft tissues in the area of the pulp chamber were removed. Further, the obtained samples were divided into 4 groups.
Fig. 1. Plates made from extracted teeth.
Each group included 12 records. The groups differed in the exposure time of the samples in a mixture of potassium and sodium salts of EDTA 17%. For the samples of the first group, no processing was carried out, the samples of the 2nd group were processed for 1 minute in a solution of EDTA salts of 17%, the materials of the 3rd group for 5 minutes, the 4th group for 10 minutes. After processing, all samples were washed under distilled water for 1 minute. Further, to prepare for examination under the FEI Quanta 250 scanning electron microscope (USA) with an attachment for X-ray microanalysis (X-ray detection unit of the BDER-K type - energy-dispersive spectrometer EDSEDAXSEM), the samples were fixed to the tables on conductive carbon double-sided adhesive tapes, after that, an electrically conductive material (coating) – gold was deposited on the surface of the samples to a thickness of 10 nm for 20 seconds. After the sample preparation, the samples were studied at a magnification from 500 to 2400 times in a high vacuum mode with an accelerating voltage of 12.5 kV at 3 points: in the area of enamel, dentin and the pulp chamber of the teeth (Fig. 2) before and after processing the samples with a solution EDTA salts 17%, in order to obtain data on the qualitative and quantitative elemental composition of dental tissues, their evaluation and comparison of the results obtained. Microphotographs of teeth were also obtained and studied.

Fig. 2. Samples under study after sample preparation.
The study was conducted on the basis of the National Research Moscow State University of Civil Engineering.
During the statistical processing of the obtained results, it was important to display the difference in the amount of Ca in the studied samples before, at the stages and after the treatment with EDTA salt solutions (17%). The data obtained were reflected in diagrams for clarity of the results. The reliability of the obtained data is reflected in the confidence interval (CI=95%).
Quantitative indicators were assessed for compliance with the normal distribution, for this the Shapiro-Wilk test was used (the significance level was p<0.05).
To describe quantitative indicators, the calculation of arithmetic mean values (M) and standard deviations (SD) was carried out. The result is presented as M + SD. Statistical analysis was performed using Statistica 12.0 software (StatSoft Inc., USA).
The analysis of the results of this study showed the presence of the following chemical elements in the studied samples: Ca, K, Mg, O, P, Na. The average percentage of elements in all samples was as follows Ca - 24.65% ± 1.9, K - 1.61% ± 1.35, Mg - 1.61% ± 0.9, O - 49.38% ± 2.1, P - 20.47% ± 1.1, Na - 32.5% ± 0.9. (Fig. 3).
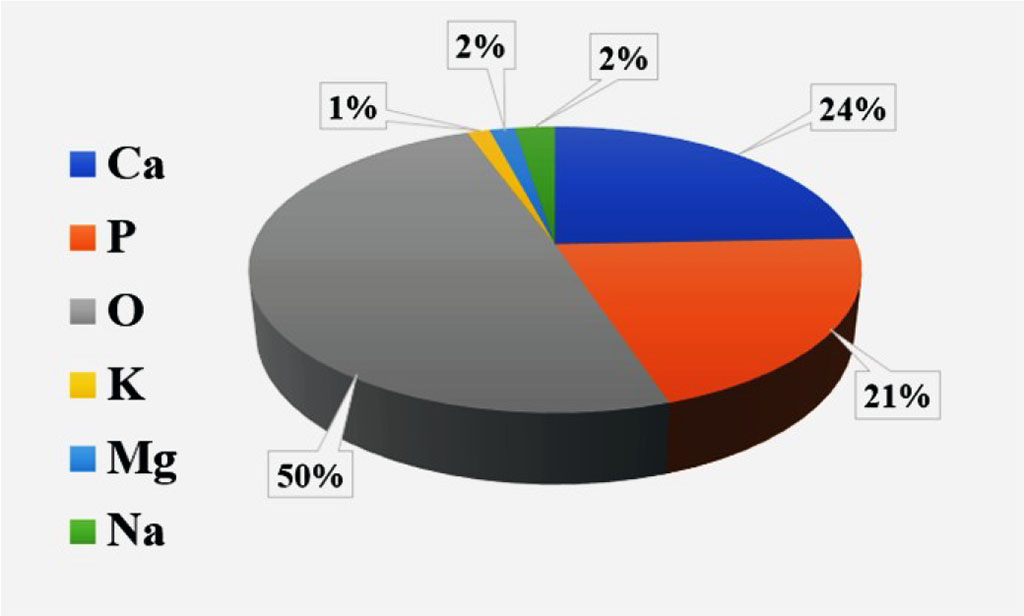
Fig. 3. The average percentage of chemical elements in the studied samples.
The average percentage of Ca in enamel - 39.1% ± 1.5, dentine - 36 1%±1.7, dentin at the border with the pulp chamber - 24.8% ± 1.3 (p<0.05).
The indicator of the percentage of Ca among the samples of the first group was 30.1% ± 1.4; samples of group 2 - 35.3% ± 1.8; 3rd group - 36.2% ± 1.2 and 4th group - 34.07% ± 1.6 (p<0.05) (Fig. 4).
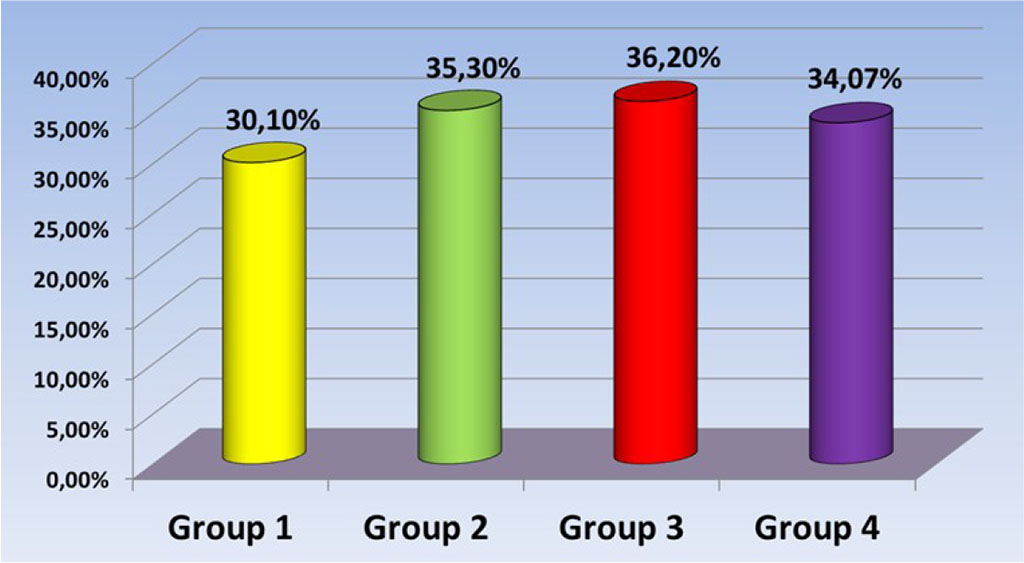
Fig. 4. Ca percentage level in test samples.
In a comparative analysis of the amount of Ca in the studied areas of the tooth tissues of the studied samples, the following data were obtained: The average percentage of Ca in the studied samples of the 1st group: in the enamel area was - 42.32% ± 1.1; dentine – 42.25% ± 1.3; dentin at the border with the pulp chamber - 29.71% ± 1.5; group 2: in the enamel area - 48.95% ± 0.9; dentine - 37.39% ± 1.2; dentin at the border with the pulp chamber - 19.32% ± 1.1; group 3: in the enamel area - 39.3% ± 1.9; dentine 38.3%±1.4; dentin at the border with the pulp chamber - 31.06% ± 1.1; 4th group: in the enamel area - 37.85% ± 0.8; dentine 37.8% ± 1.5; dentin at the border with the pulp chamber - 26.6% ± 1.2 (Fig. 5).
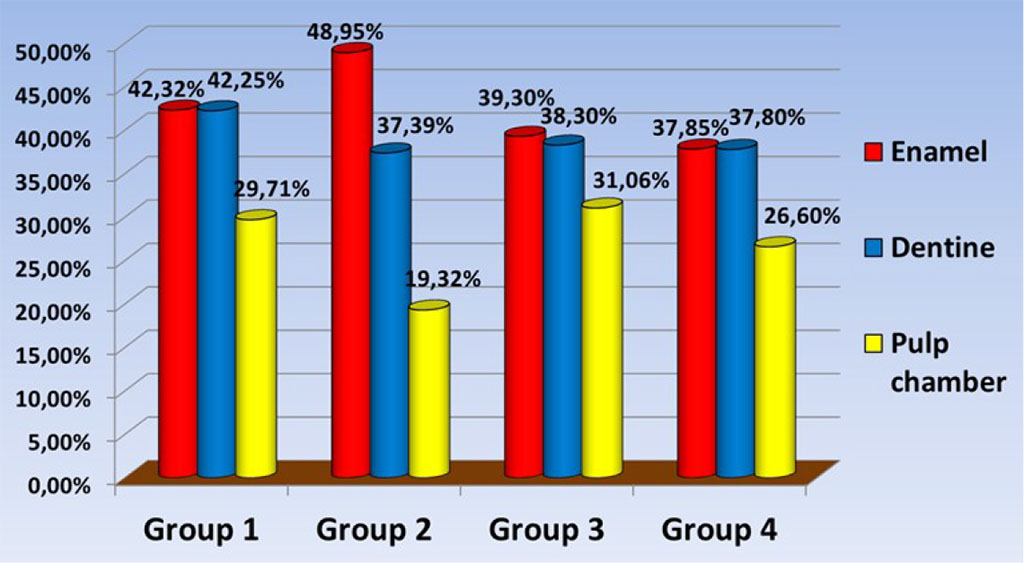
Fig. 5. The amount of Ca in the study samples.
According to the results of statistical processing, the values that were gross errors based on the Grubbs criteria were excluded. The average values of the Ca content of significant differences and, as a result, the absence of a decrease in the quantitative content of Ca in the studied samples, which may indicate the absence of the process of tissue demineralization.
When studying electron micrographs of dental tissues in order to open the dentinal tubules after processing the samples with a 17% EDTA salt solution, we revealed the presence of a smeared layer in the samples of all groups in the dentin area, which indicates the absence of an effect of a 17% EDTA salt solution on the smeared layer (Fig. 6) obtained during mechanical processing of tooth tissues during the preparation of samples for further use in clinical practice. In the area of the pulp chamber, in places of natural depressions, where mechanical treatment with a separation disc and diamond burs was not carried out, dentinal tubules were visualized without the presence of a smeared layer (Fig. 7).
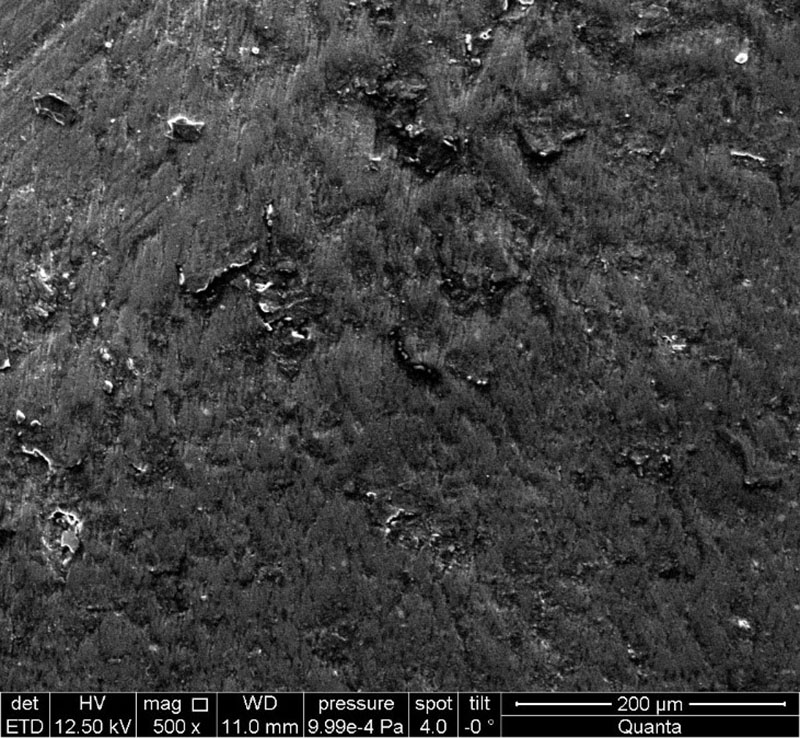
Fig. 6. The presence of a smear layer in the dentinal tubules. Electronic micrograph (×500).
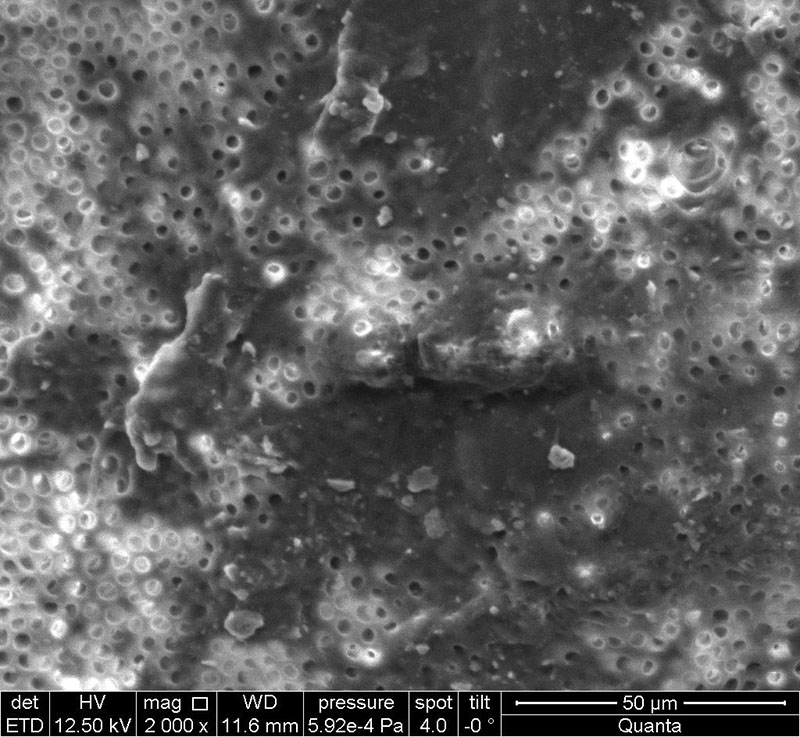
Fig. 7. No smear layer in dentinal tubules. Electronic micrograph (×2000).
Based on previous studies, it can be concluded that a fully or partially demineralized dentin matrix contributes to a reduction in the period of bone formation compared to mineralized dentin. In addition to acid solutions (HCl, HNO3, citric acid), which can cause chemical burns of tissues, and based on studies for endodontic practice and the fact that EDTA salt solutions can affect the process of demineralization of tooth tissues, we assumed that the most commonly used in endodontic practice, to expand and facilitate the passage of root canals, the most affordable EDTA salt solution in Russia (17%) can affect the acceleration of the integration of dentin grafts by demineralization [12-14], so the solution of choice in our study was the drug this particular concentration. When determining the exposure time, we proceeded from the recommendations for the use of the solution for endodontic treatment.
The use of autografts in the form of plates, in contrast to dentin crumbs, clinical cases of which are demonstrated by the KometaBio Company, using a 10% EDTA solution for 5 minutes to prepare autografts [11], expands the possibilities of performing reconstructive operations in the oral cavity, but despite an increase in concentration of the applied solution to 17% and an increase in the exposure time, it was not possible to obtain the results of dentin demineralization in the framework of our study. Confirmation of the proposed hypotheses about the possibility of decalcification of grafts from dentin would allow using this protocol for processing dentin grafts directly during surgery. The lack of decalcification of the studied samples may be due to insufficient exposure time, however, an increase in exposure time intervals will make the procedure difficult to implement as part of a surgical operation. An increase in the operation time may lead to the development of purulent-inflammatory complications, which makes the procedure unsuitable for use directly during the operation.
Thus, treatment with 17% EDTA salt solution for up to 10 minutes exposure of dentin autografts used in the form of plates does not significantly affect their microstructure, qualitative and quantitative composition (CI = 95%), and the degree of demineralization, which makes carrying out this manipulation intraoperatively in alveolar bone plasty is impractical.