- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 3: e1. DOI 10.35630/2199-885X/2022/12/3.13
Diagnosis of terminal conditions is an important field of forensic medicine. When shock occurs all human physiological functions are replaced with pathological processes, including development of disseminated intravascular coagulation (DIC) syndrome, develop. In DIC syndrome two opposite processes, such as increased blood clotting and activation of the fibrinolytic system, take place. This paper provides information on post-mortal diagnosis of DIC syndrome for anaphylactic and other types of shock.
The quantitative content of the fibrinogen fraction and the results of paracoagulation tests allow post-mortem diagnosis of DIC syndrome and identification of the process stage. These studies indicate shock at the time of death and are especially relevant for post-mortem diagnosis of anaphylactic and other types of shock.
Keywords: DIC syndrome, fibrinolysis, shock, post-mortal diagnosis.
Diagnosis of terminal conditions is an important field of forensic medicine. When shock occurs all human physiological functions extinct and pathological processes, including development of disseminated intravascular coagulation (DIC) syndrome, develop. [1, 2]. In DIC syndrome two opposite processes - increased blood clotting and activation of the fibrinolysis system - develop. A number of indicators including determination of fibrinogen (total, by thrombin) are used for intravital diagnostics of DIC syndrome [1, 2, 3], however these methods can’t be used with cadaveric blood.
The mechanism of post-mortal fibrinolysis (fibrinogenolysis) is not completely clear until today. Italian anatomist Morgagni (1761) noticed that cadaveric blood does not clot in case of sudden death. Hanter (1786) and Denis (1838) described the phenomenon of sudden dissolution of blood clots by 12-24 hours after taking blood from corpses veins. Dastre (1893) explained this process by decomposition of fibrin and called it “fibrinolysis”. The interest in detailed research of corpses blood started in the 30s of the XX century, which was associated with the development of transfusiology and secondary «discovery» of fibrinolysis. In case of sudden death at first a lot of friable red clots were formed, which dissolved within a short period of time; and the ability to secondary blood coagulation was completely lost [1].
Sulfitolysis method to determine the fibrinogen fraction and a number of paracoagulation tests - ethanol test, protamine-sulfate and β-naphthol test - were proposed for the postmortem diagnosis of DIC syndrome.
A significant increase and a significant decrease of fibrinogen fraction content were observed in the first stage and the third stage of DIC syndrome, respectively. Paracoagulation tests increased with the severity of the syndrome [4]. The sulfitolysis method specifically precipitates fibrinogen and related molecules , which have chains and degradation fragments of fibrinogen in their structure [5].The precipitation of other protein blood fractions also occurs [6]. So, the term “fibrinogen fraction” seems to be more correct than “fibrinogen”, when we are talking about serum of cadaver blood. To assessing fibrinogen fraction content in blood serum it is necessary to know standard values. There is no unambiguous definition of “standard”. It is usually a conditional concept, when a certain indicator in the control group is compared to an indicator in a study group with definite diseases[7]. The content of fibrinogen fraction in cadaver blood serum, which is determined by this method, is believed to be up to 12 g/l [4].
A study of cadaver blood serum of 4 women, who died as a result of anaphylactic shock during treatment (analgetics and antibiotics injection), was performed. These samples formed the first group. The second group included blood samples of 10 people, who died as a result of acute burn injury in an emergency car, in the admission department or in emergency unit on the first day after burn injury (burn shock). The third group included blood samples of 8 people who died in the burns department as a result of burn disease. The first group of comparison included blood samples from 35 people who died suddenly as a result of acute myocardial infarction (AMI). The second group of comparison included blood samples from 8 people who died in situ as a result of combined injuries in road traffic accidents.
Determination of fibrinogen fraction was performed using the Rampling M.W., Gaffney P.I. sulfitolysis method, which was modified by us taking into account the researches of other authors [4, 5, 6, 8]. 0,1 ml of serum was added to 0,9 ml 10,9% sodium sulfitis, shaken, kept in a thermostat at 37˚С for 15 minutes, centrifuged 15 minutes at 1600 g. Supernatant was decanted. Sediment was rinsed with adding 0.9 ml sodium sulfite solution, and centrifuged again under the above conditions. Supernatant was decanted and a sediment was hydrolysed in 5,0 ml solution of 4 M urea in 0.1 N sodium hydroxide in a thermoblock at 100˚С for 15 minutes, than cooled down. The optical density of the solution was measured on an SF-2000 spectrophotometer in 0,1 cm quartz cuvettes at wavelength range of 210-300 nm against the solvent.
The calculations were carried out using the formula: С = 30,6 х D х Р,
where С – concentration of fibrinogen fraction in sample (mg/ml);
D – optical density of hydrolysate at the maximum point (on the average wavelength is 224-226 nm); 30,6 – dependency ratio of optical density by the content of fibrinogen’s fraction,
P - coefficient of additional dilution. Spectrogram is shown in the figure (fig. 1).
Determination of soluble fibrin monomer complexes (SFMC) was performed by paracoagulation tests [2, 9, 10].
Ethanol test. 100 mkl of the serum was poured in the microtube, 35 mkl of 50% ethanol was added, shaken and left for 15 minutes at room temperature. after that evaluated the result. The test was considered to be positive if a gel formed.
Protamine-sulfate test. 100 mkl of serum was poured in the microtube, 25 mkl of 1% protamine-sulfate solution was added, shaken and left for 15 minutes in thermostat at 37˚С. After that the results were evaluated. The reaction was considered to be positive if a white clot (or several clots), consisting of filaments or flakes of fibrin, was formed at the bottom of the tube. If the reaction was negative, the solution was gritty or cloudy.
There were no significant differences in the content of the fibrinogen fraction in the study groups, except for the group with burn disease. At the same time large intervals of data were found in each group of observations (table 1).
Table 1. The content of the fibrinogen fraction in serum of cadaveric blood
| Parameter | Acute myocardial infarction | Mechanical injury | Anaphylactic shock | Burn shock | Burn disease |
| n | 35 | 8 | 4 | 10 | 8 |
| M ±
m (г/л) |
16,7 ± 1,9 | 16,6 ± 4,1 | 30,3 ± 8,6 | 22,5 ± 3,7 | 2,9 ± 1,2 * |
| min –
Max (г/л) |
0,0 – 38,8 | 4,3 – 33,9 | 19,3 – 55,9 | 10,1 – 49,0 | 0,0 – 9,6 |
| * - reliability р < 0,001 versus group of acute myocardial infarction |
|||||
At death, as a result of anaphylactic shock, the content of the fibrinogen fraction was significantly increased. The minimum content of the fibrinogen fraction in cases of anaphylactic shock was 19,3 g/l, which is significantly higher than the lower indicator in any other observation group (table 1).
In the group where death occurred as a result of anaphylactic shock, the fibrinogen fraction content was sharply increased. The minimum content of fibrinogen fraction was in cases of anaphylactic shock turned out to be 19.3 g/l, which is significantly higher than the lower indicator in other observation groups (Table 1).
In thermal trauma, burn shock is the cause of mortality in 21% – 25% of cases [11]. In our studies, the content of fibrinogen fraction in blood serum in acute burn injury (burn shock) turned out to be almost an order of magnitude higher than in burn disease. In acute burn injury the minimum content of fibrinogen fraction was 10.1 g/l, and the maximum content of this fraction in burn disease was 9.6 g/l. The results obtained are consistent with the literature data on the development of DIC syndrome in the acute period of burn injury [12]. Paracoagulation tests were positive in 8% of all cases. The ethanol test was positive in three cases only: 2 – for mechanical injury and 1 – AMI. The protamine-sulfate test was positive in 3 cases – 1 for acute burn injury (burn shock) and 2 for anaphylactic shock. Consequently, despite the various variants of death cause, the fatal outcome of critical conditions in most of the victims was accompanied by the development of shock. Basically, the development of DIC syndrome was limited to the development of the first stage, since the positive results of paracoagulation tests begin to manifest from the second stage.
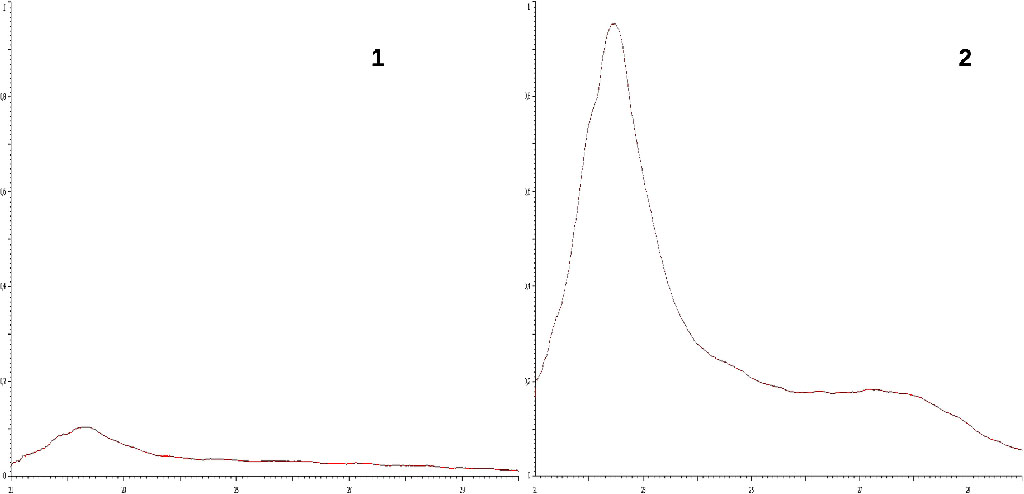
Fig. 1.
Spectrograms of cadaveric blood serum fibrinogen fraction.
1 –
«norm», 2 - DIC syndrome
X-axis:
wavelength (210 – 300 нм).
Y-axis: optical density (D = 0,000 – 1,000).
The content of fibrinogen fraction exceeding 10 g/l (presence of DIC syndrome) in the comparison groups was found in 62% of cases with mechanical injuries (traffic accidents) and 57% with AMI, which indicates traumatic and cardiogenic shock in the antemortal period. The development of traumatic shock indicates a period of survivability after the injury. It is known that the presence of DIC syndrome is detected in all patients with AMI admitted to the hospital [13]. An acute ischemic attack is also known to develop in the first 15 minutes and most often leads to death. Then, the stage of reperfusion occurs, which also causes even greater myocardial damage [14], but a person can survive, and such people are admitted to a hospital. Thus, the development of DIC syndrome in AMI is caused in most cases by the presence of cardiogenic shock.
The quantitative content of the fibrinogen fraction and the results of paracoagulation tests allow post-mortem diagnosis of DIC syndrome and identification of the process stage. These studies indicate shock at the time of death and are especially relevant for post-mortem diagnosis of anaphylactic and other types of shock.