- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2022. 12; 3: e1. DOI 10.35630/2199-885X/2022/12/3.9
Here we describe the course and specific aspects of the treatment for myocardial infarction with left coronary artery occlusion after bilateral multisegmental pneumonia. Acute left coronary artery trunk occlusion is a life-threatening condition with high mortality. The paper describes the most probable mechanisms of myocardial injury after COVID-19, and shows how percutaneous intervention, mechanical circulatory support and the correct choice of drug therapy can improve clinical outcome.
Keywords: COVID-19, acute coronary syndrome, left coronary artery trunk occlusion
Acute COVID-19-associated cardiovascular syndrome is characterized by a wide range of cardiovascular and thrombotic complications of coronavirus infection. COVID-19 can induce instability of atherosclerotic plaque with subsequent development of myocardial infarction (MI), significantly increasing the risk of death of infected patients.
Acute coronary syndrome due to the left coronary artery (LCA) trunk occlusion is a life-threatening condition with high mortality. Acute complete occlusion of the LCA trunk is quite rare and leads to the sudden death or cardiogenic shock with a high, up to 80 %, hospital mortality. LCA trunk supplies blood to the 75 % of the left ventricle (LV) in patients with RCA dominance or with ‘balanced’ type of blood supply and 100 % in the case of LCA dominance. According to the published data, the incidence of lesions of the LCA trunk is 5–12 %, depending on whether it was occlusion or stenosis of the LCA trunk, as well as ST segment elevation/non-ST segment elevation myocardial infarction (MI) [1].
Case description. An ambulance team delivered a patient R., male, 64 years old, to the department of cardio- and X-ray-vascular surgery. The patient had severe constricting pain behind the sternum about 3 hours ago, as well as general weakness and shortness of breath in the supine position. The CHD was newly diagnosed, and approximately 2 months ago he had COVID-19-associated bilateral multisegmental pneumonia.
The patient started smoking by age 30 (10 pack/years).
At the initial examination, the patient’s condition was of moderate severity, he was fully conscious, and his skin and visible mucous membranes were pale, with acrocyanosis. On auscultation, bubbling rales were heard in all segments of lungs, breathing rate — 24 per minute, SpO2-70 %. No signs of fever. Blood pressure: 80/65 mmHg, heart rate: 130–140 bpm. The abdomen has typical shape, participates in the act of breathing, soft and painless on palpation. Liver and spleen are not enlarged. No signs of peripheral edema.
Urgent laboratory testing, electrocardiography (ECG) and coronary angiography were conducted.
ECG: Q-MI of the anterior LV wall, atrial fibrillation with a ventricular rate of 150 beats per minute. Express test for COVID-19 was negative. Complete blood count, Hb — 176 g/l, red blood cells — 5.44*109/L, white blood cells — 6.5 g/l, ESR — 3 mm/h. Blood biochemistry: glucose — 29.4 mmol/l, urea — 7.5 mmol/l, creatinine — 220.8 µmol/l, ALT — 108.9 U/l, AST — 374.8 U/l, potassium — 3.7 mmol/l, sodium — 143 mmol/l, chlorine — 107.0 mmol/L. Acid-base balance: pH — 7.16, p O2 — 49, p CO2 — 34.2.
Urgent angiography showed occlusion of the LCA trunk without signs of antegrade contrast enhancement of the left coronary artery distal to the occlusion.
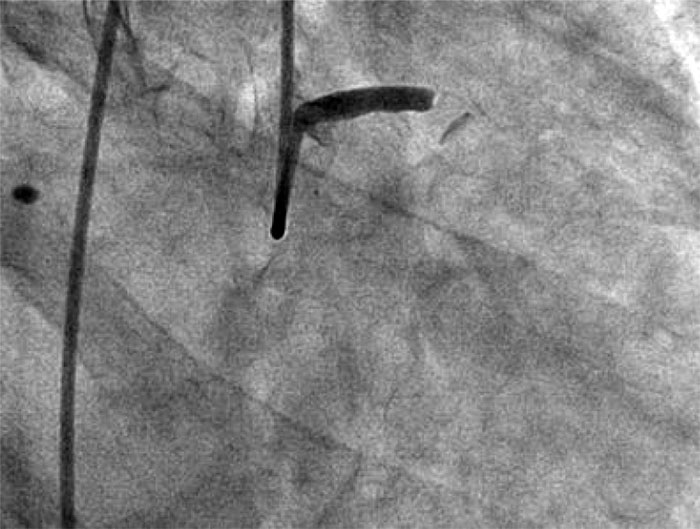
Fig. 1. LCA trunk occlusion
LCA trunk stenting was performed, antegrade blood flow — TIMI 3. The patient was transferred to the intensive care unit (ICU) for treatment with high doses of adrenergic agonists and intra-aortic balloon counterpulsation (IABC) 1:1. Within 90 minutes, the patient was transferred to the operating room for angiography of the left lower limb, as he had significant pain in the left leg without effect from opioids, and he had no pulsation over the popliteal artery and the dorsal pedis artery. Angiography showed thrombotic occlusion of the left common iliac artery.
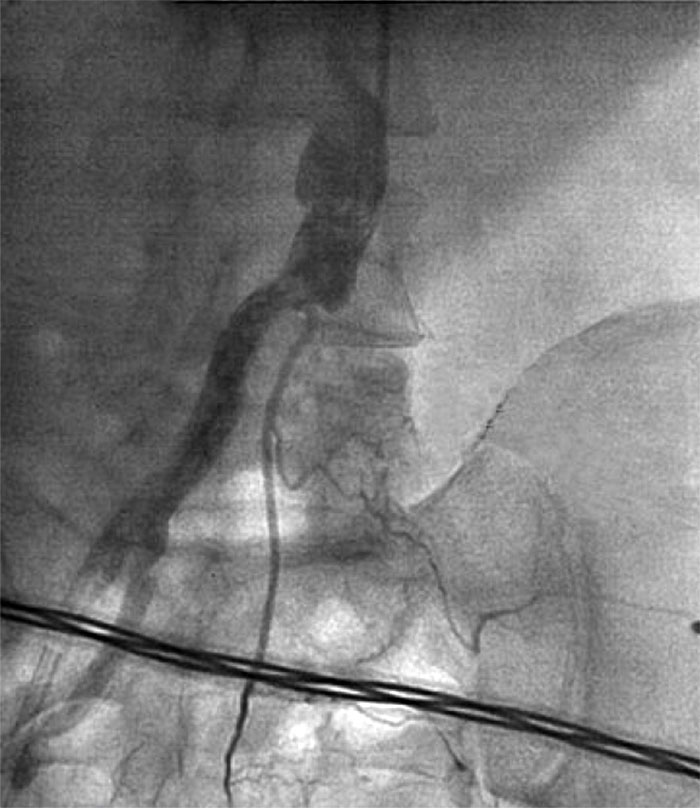
Fig. 2. Left iliac artery occlusion
Stenting of the common and external iliac arteries on the left was performed. The patient was transferred to the intensive care unit. After revascularization of the coronary arteries and resolution of the pulmonary edema, as well as on the background of drug correction of metabolic acidosis, laboratory tests were as follows: pH — 7.42, p O2 — 96, p CO2 — 34.0. Over the first three days after myocardial revascularization, non-invasive ventilation sessions were conducted. 3 days after surgery, IABC was removed, on the 8th day the patient was transferred to the department of cardio- and X-ray-vascular surgery. Echocardiography data: EF — 35–37 %, EDV — 227 ml, ESV — 147 ml. Diffuse reduction in the left ventricular myocardial contractility. Pulmonary artery pressure is 58 mmHg. Bilateral hydrothorax: 500–700 ml on the right, approximately 1 l on the left. Pleural drainage was performed. Computed tomography (CT) showed signs of pulmonary hypertension. Interstitial changes in lungs are due to edema (interstitial and alveolar) in combination with an inflammatory process of viral etiology. Bilateral hydrothorax. Moderate mediastinal lymphadenopathy.
The patient received treatment of heart failure with angiotensin converting enzyme inhibitors (Ramipril 1.25 mg in the morning under BP monitoring), cardiac glycosides (digoxin 0.025 µg, ½ tab at noon), long-acting nitrates (Kardiket 10 mg BID under BP monitoring), aldosterone receptor inhibitors (verospiron 100 mg in the morning), loop diuretics (Torasemide 10 mg in the morning). The patient also received anticoagulants (Clexane 0.8 ml BID subcutaneously), antiaggregants (Cardiomagnyl 75 mg in the evening, Clopidogrel 150 mg at noon), proton pump inhibitors (Nolpaza 20 mg BID), lipid-lowering drugs (Atoris 80 mg in the evening). For blood glucose correction, short-acting insulin was administered according to the glycemic profile. Anticoagulant therapy was discontinued on the 10th day after surgery. Starting from the 1st day after hospital admission, the patient received Amoxiclav 1.0 intravenously TID, which was replaced with Vancomycin 1 g intravenously BID on the 10th day, and on the 20th day — with Meropenem 500 mg intravenously TID in combination with Moxifloxacin 400 mg OD orally. On 17th day after hospital admission fever was noted without previous episodes of body temperature elevation. On the 13th day, shortness of breath after minimal physical exertion was noted. it was considered to be due to the sequelae of bilateral pneumonia, low ejection fraction and significant mitral insufficiency. ECG: sinus rhythm, 65 BPM, without ischemic changes, SpO2 — 96 %. On the 15th day of hospital stay, general weakness and shortness of breath have increased. ECG — without changes. BP — 80/40 mmHg, SpO2 — 95 %. Later, ventricular fibrillation occurred. Defibrillation was performed twice. For resuscitation purpose, a bolus of a tissue plasminogen activator (15 mg) was administered. Hemodynamics was stabilized by high doses of adrenergic agonists. IABC 1:1 has been established. Urgent coronary angiography showed recanalization of stent thrombosis in the LCA trunk, which have recurred after multiple balloon angioplasty procedures. Stenting of the bifurcation of the LCA trunk was performed. The patient was transferred to the ICU. Heparin was prescribed, with dose adjustment according to the coagulation profile, Clopidogrel was replaced with Ticagrelor.
On the 39th day the patient was discharged with improvement for cardiology follow-up care of at the local clinic. 1 month after discharge, control echocardiography showed following results: PV — 40 %, EDV — 231 ml, ESV — 141 ml, grade 2–3 mitral insufficiency. Diffuse reduction in myocardial contractility of the left ventricle. Pulmonary artery pressure is 56 mmHg.
Discussion of the results. Post-COVID-19 cardiovascular complications often develop after stabilization and/or improvement of the respiratory status of the patient. It was assumed that cardiotropic viruses like SARS-CoV-2 can persist in myocardial tissues for several weeks or even months [2].
Considering the clinical course of the disease, it should be noted that this patient was prone to thrombophilia. Based the historical and chest CT, the patient had COVID-19-associated bilateral multisegmental pneumonia, which significantly complicated the course of MI. Although the mechanism of myocardial injury in COVID-19 has not been fully studied, in this clinical case, the most probable mechanisms were hypercoagulation due to endothelial dysfunction, platelet and Willebrand factor hyperactivity, increased production of the type 1 tissue plasminogen activator inhibitor and reduced production of tissue plasminogen activator causing fibrinolysis, which has led to the abnormal blood flow and micro- and macrothrombosis, severe hypoxemia with induction of anaerobic processes, intracellular acidosis and oxidative stress, an imbalance between the myocardial oxygen demand and its delivery against the background of viral inflammatory condition, hypoxia, oxidative stress, endothelial damage and hypercoagulation [3, 4, 5].
In addition to endovascular intervention and drug therapy, methods of auxiliary blood circulation (extracorporeal membrane oxygenation, IABC) are indispensable, which are the methods of choice in cardiogenic shock caused by acute left ventricular insufficiency with thrombotic occlusion of the LCA trunk.
As for antiaggregant products, for example, in PLATO study, a comparative analysis of Ticagrelor and Clopidogrel was carried out. The total incidence of vascular death, myocardial infarction and stroke was lower in Ticagrelor arm vs. Clopidogrel arm: 9.8 % and 11.7 %, respectively. The advantages of Ticagrelor over Clopidogrel persisted regardless of the initial diagnosis and treatment strategy. Based on this clinical case, it can be concluded that in patients with MI complicated by cardiogenic shock, with thrombotic occlusion of the LCA trunk, and with a novel coronavirus infection, Ticagrelor is the drug of choice.
It should be noted that the Guidelines for diagnosis and treatment of ST-Segment Elevation Myocardial Infarction do not provide distinct indication of the duration of the use of direct anticoagulants in thrombotic occlusion of the LCA trunk for the prevention of cardioembolic complications. Nevertheless, with the expansion of the ambulatory regimen against the background of double antiplatelet therapy and normalization of the patient’s general condition, enoxaparin was discontinued on the 10th day of the postoperative period, which may have caused stent thrombosis and led to the repeated PCI. After repeated stenting, anticoagulants were discontinued on the 7th day against the background of treatment with Ticagrelor.
The lack of ischemic changes on ECG after repeated deterioration of the patient’s condition do not allow to completely rule out stent thrombosis. The increasing respiratory distress, which did not relieve after provision of humidified oxygen, was the equivalent of angina pectoris. Initially, hemodynamic parameters did not change with deterioration, but subsequently hypotension and ventricular fibrillation occurred, which required immediate resuscitation and control angiography.
Thus, performing PCI as early as possible in patients with acute LCA trunk occlusion with the use of mechanical circulatory support and the correct choice of antiplatelet agents and anticoagulants, as well as the duration of their administration, allows to maximize the clinical outcome, especially in patients with a history of bilateral COVID-19-associated multisegmental pneumonia. In case of deterioration of the patient’s general condition, but in the absence of changes on the ECG and respiratory distress, it is necessary to rule out stent thrombosis, which can show signs of respiratory failure due to bilateral multisegmental pneumonia in combination with heart failure.