- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
One of the priorities of modern medicine is the development of biomaterials for tissue engineering, transplantation and vascular surgery. Such materials should facilitate the regeneration of damaged body organs and tissues. The problem of regeneration of wound lesions affecting various skin and soft tissues is related to a high rate of secondary infection, deep metabolic shifts in the conditions of severe inflammations, activated oxygen-independent phagocytosis and intensified free-radical response. In patients with wounds of various etiologies, modern clinical practice widely employs numerous wound dressings, where properties are specified in advance according to the stage the wound is going through.
The principle of a wound coating is the provision of a moist abacterial environment, which is optimal for accelerated healing. The subjects of our experimental studies were 45 white laboratory rats (males, outbred, each weighing 250-300 g) with soft tissue wounds developed by a specifically designed method. As materials for wound treatment we used chitosan-based multilayer wound dressings of different structure and porosity degree. They were introduced into simulated wounds in the back area between the shoulder blades in rats. The experimental part of the study focused on the sorption capacity of the wound exudate obtained after extraction and weighing of the samples within the control time. The laboratory part of the experiment involved studying the sorption capacity of wound dressing samples in relation to distilled water and blood serum.
The morphological assessment of the dressing samples surface was performed on a Helios NanoLab 600electron-ion scanning microscope. It was observed that chitosan-based multilayer wound dressings, had different morphological parameters and molecular structure as well as high sorption activity. Chitosan samples with a so-called loose structure and high porosity should be used in a clean (aseptic) wound at the initial stages of treatment, with no inflammation, where initially high adhesion to surrounding tissues is required, thus, ensuring tightness and keeping a blood clot within the wound. Chitosan samples with a tight-packed structure and a smaller diameter of pores showed good results in purulent inflammation (exudative phase) with a large amount of wound exudate. Due to its dense outer layer, their structure is capable of retaining the skeleton functions for a long time, thus offering effective drainage of the wound.
Keywords: chitosan; wound; wound process; wound dressing; wound treatment; preclinical studies; regenerative medicine; sorption activity; biotechnology.
Healing skin and soft tissue injuries with surgical and conservative methods has still remains an issue that is not yet completely solved. The main obstacles to epithelialization and granulation are tissue dystrophy, oxidative damage, wound moisture imbalance, infection and other complications, which affect the area of surgical resection, injury or burn. The development of scar tissue or of any other structural change at the wound site has a negative effect on the patient’s life quality [1-3].
There are a number of available techniques are aimed at intensifying wound healing and improving the structural and functional properties of the newly developing tissue. Most of these techniques are based on wound coatings of different composition and functional specifics [4-6].
Wound dressings are composite systems with varying degrees of complexity and standing as a combination of a bandage and a medication that can promote healing significantly. The areas of application for wound coatings are extremely diverse and stay in line with the principles of pathogenetic therapy. This type of dressing are used to treat joint pathology, heat burns, radiation lesions involving skin and mucous membranes, purulent wounds and even trophic ulcers. Wound dressings developed nowadays are also widely used in surgical dentistry, maxillofacial surgery, periodontics and in diseases of oral mucosa. The main objective of the wound dressings is creating the conditions to accelerate the wound healing through all of its stages, and reduce the hospitalization time [7-18].
The wound healing process is a sequence of stages, ultimately leading to epithelialization and closure of the skin lesion. Each stage of wound healing has its own features. The inflammation stage can be described by the development of a large amount of exudate; the proliferation stage requires regeneration stimulation and reduction of wound injury to prevent the destruction of young granulation tissue. As for the healing stage, this is where young granulation tissue is transformed into tough scar tissue, which means the need for accelerated cell migration and cell division. Each stage requires choose a certain type of wound coatings, given their most significant properties. Assess the course of the wound process will take a visual assessment of the wound surface, as well as studying specific markers related to inflammation [19-21].
Modern medicine has, at its disposal, a range of new-generation biodegradable synthetic and natural materials. Chitosan, among them, stands out due to its structure and properties; as chitosan-based materials are close to human body tissue cells [22].
There is evidence of vast potential effect that can be achieved by developing new chitosan-based biotechnological wound dressings capable of changing their physical, chemical and strength properties – from a hydrogel to a dense frame structure with varying degrees of swelling and water absorption. Chitosan-based wound dressings offer a number of advantages: biological compatibility with body tissues; bacterial staticity. It possesses enhancing capacity for boost regeneration during wound healing; minimal number of side effects; high wound-healing activity; moisture and air permeability; high porosity and moisture absorption; mechanical stability along with the material plasticity; a controllable period of biological resorption within the body; the ability to develop a film; possible use as a depot carrier for medications. Gradual biodegradation of chitosan in the alteration zone, along with diffusion through the swollen walls of the hydrogel, facilitates gradual release of the immobilized pharmacological agent through controlled time intervals, followed by replacement with native cells and tissues. Thus, it provides a stage-by-stage prolonged medication therapy against pathophysiological processes going on in the wound [23-25].
Due to their high adhesive properties and significant antiseptic and plastic activity, chitosan-based wound dressings are effective in local treatment of burn wounds. This achieved by accelerated development of granulation, fibrous connective tissue, and a decrease in the thickness of the skin crust. The very principle of using wound dressings relies on wet wound healing. Regeneration is more active in an abacterial moist environment, which promotes greater migration of epithelial tissue cells to the inflammation focus, and leads to activated endogenous proteases, whereas reducing the use of allergenic proteolytic enzymes [26].
A detailed study of the sorption properties of chitosan-based multilayer wound dressings with different structures and porosity degrees would offer a reliable explanation to their applications during various phases of wound healing.
Aim of the study: to investigate the sorption activity of multilayer chitosan-based wound dressing and to assess their possible applications through the stages of wound healing.
The experimental samples used in this study were chitosan-based core-shell type multilayer wound dressings. They were developed at the Kurchatov Institute (Moscow, Russia. Their molecular weight – 600 kDa; porosity – 98%; pore size: core − 20-45 microns; shell − 70-200 microns. The porous materials were obtained employing the technology of chitosan solution lyophilic drying. The experiment was aimed at studying the sorption activity of two samples (sample A and sample B with different morphological, physical and chemical parameters.
Sample A possesses a loose structure and a high degree of porosity, whereas sample B featured a denser structure and a smaller pore diameter. The reference material was a sample of a textile gauze bandage (density – 36 g/m2; that is a medical sterile bandage. All studied samples had the same volume (V= 0.125 cm3).
The experimental part of the study involved 45 white outbred laboratory rats, male, weighing 250-300 g. The animals were kept within the requirements for standard vivarium conditions. The air temperature was maintained at 20-24° C; humidity – 45-65%; light mode – 12 hours of light VS. 12 hours of darkness. The animals were given 14 days for acclimation.
The animals were kept in cages, individually, with free access to food and water. The experiments were carried out in following strictly the European Council Directive on ethical principles in working with laboratory animals (the European Council Directive (86/609/EEC)) and Directive 2010/63/EU of the European Parliament and of the Council of the European Union. There were three experimental groups – 15 animals in each. The models of soft tissue wounds in experimental animals were made with a specially developed technique (Patent RU #2703709 of August 23, 2018). The technique consisted of creating a wound with specified parameters, this done by introducing a hydrophilic polymer implant of spherical shape into soft tissues for 6-7 days. The implant was inserted through a layer-by-layer incision of soft tissues down to the required depth. Further on, the wound was sutured in layers for the above-mentioned period. The implant was removed surgically, and then the tested material was introduced into the cavity. A sterile cloth with Levomekol ointment was applied on top of the studied materials to be further fixed with separate nodular sutures to the wound edges (Figure 1).
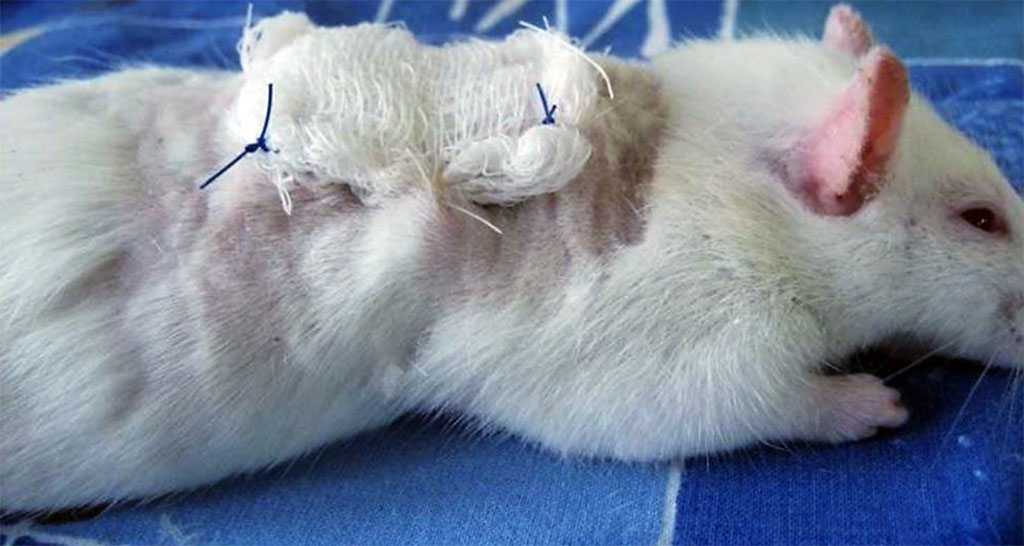
Figure 1. Fixation of a sterile napkin with Levomekol ointment to the wound edges of a laboratory animal.
All experimental surgical interventions on laboratory animals were done under aseptic conditions and under general anesthesia. The pathology induction (surgical intervention implying the introduction of a hydrophilic polymer implant and wound coatings into soft tissues) was performed under anesthesia, namely, Zoletil 100 (Virbac, France; 30 mg per 1 kg of body weight, intramuscular introduction). Prior to applying wounds, the fur was cut on the back between the shoulder blades with scissors at the site of the surgery. The remaining fur was removed with a depilation cream (Eveline Cosmetics, Poland), which was applied for 3 minutes. After that, the surgical spot was treated once with a 5% alcohol solution of iodine and 70° ethyl alcohol. A sterile Dermopunch skin biopsy punch (diameter – 5 mm, by Sterylab, Italy) was used to create 2 full-layer skin wounds with a depth up to the superficial fascia of the muscles, this done through a pulled skin fold on the back between the shoulder blades. The wounds caused no discomfort to the animals, nor did they affect their activity or appetite in any way. To relieve the pain syndrome through the postoperative period (Day 1), the animals had Flexoprofen injected. No euthanasia was carried out.
The sorption activity of materials was studied following the ISO 811-81 standards subject to the method of dynamic identification of tissue resistance to liquid penetration by means of hydrostatic pressure (sorption capacity). The identical wound cavities in the animals received the following wound dressings: Group 1 – a chitosan A sample; Group 2 − a chitosan B sample; Group 3 − a sample of textile material. Then, sticking to the control time allowed (15, 30, 60 minutes), the material was extracted from the wound, followed by a control weighing (Figure 2).
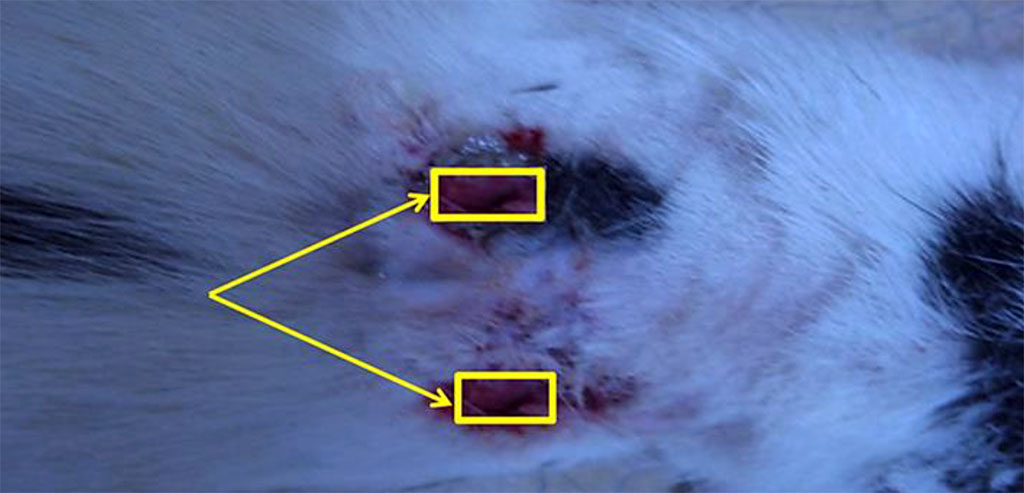
Figure 2. Wounds with the dressings inserted into them.
The laboratory part of the study involved three stages. Stage 1 – the sorption was identified by placing the samples in a unit volume of distilled water. Stage 2 – blood serum was used as a sorbent. At Stage 3, the serum protein content was detected prior to, and following sorption (Bradford colorimetric assay).
The morphological assessment of micro- and nano-relief of the chitosan-based wound coating surface on the samples was done with scanning electron microscopy on a Helios NanoLab 600electron-ion scanning microscope (FEI Ltd., USA) (Figure 3).

Figure 3. Helios NanoLab 600electron-ion scanning microscope
The statistical processing of the study results was done by Windows 10 operating system using the STATISTICA 6.1 (StatSoft, Inc., USA) and Excel (Microsoft Office 2010) software. The hypothesis of the cumulative distribution normality in the samples was tested relying on the Shapiro-Wilk and Kolmogorov-Smirnov criteria. The statistical significance level was set at p<0.05. The differences between the quantitative parameters with a normal distribution were evaluated through the Student’s t-test, whereas independent samples were evaluated relying on the nonparametric Mann-Whitney test. The differences in all cases were considered statistically significant at p<0.05. The significance level of the relationship between the two qualitative variables was tested through Pearson’s Chi-square (χ2) test.
Table 1 presents the data related to wound exudate sorption capacity in the studied samples.
Table 1. Sorption capacity of wound exudate, samples, (cm3), (p<0.05)
| Samples | Time
of exposure, minutes |
||
| 15 | 30 | ||
| Chitosan, sample A | 0.178±0.007 | 0.194±0.011 | |
| Chitosan, sample B | 0.035±0.001 | 0.111±0.004 | |
| Gauze bandage | 0.161±0.002 | 0.161±0.009 | |
Studying the hygroscopic and water-repellent properties showed that the highest sorption activity was to be seen in sample A, which exceeded the sorption capacity of samples B by 5.09 ±0.18, 1.75±0.11 and 1.13± 0.06 times (exposure time – 15, 30 and 60 minutes), respectively, and comparing to the textile sample (gauze bandage) − 1.11± 0.03, 1.20±0.06 and 1.21± 0.04 times (15, 30 and 60 minutes), respectively.
The distilled water and blood serum sorption indicators, which reveal the amount (volume) of the substance that can be absorbed by the wound dressing of a specified volume, can be seen in Table 2.
Table 2. Sorption capacity of distilled water and blood serum in relation to the specified volume of the studied samples, (cm3), (p<0.05)
Samples |
Time of exposure, minutes | |||||
| 15 | 30 | 60 | ||||
| Distilled water | Blood serum | Distilled water | Blood serum | Distilled water | ||
| Chitosan, sample A | 0.283±0.009 | 0.103±0.005 | 0.364±0.008 | 0.138±0.003 | 0.368±0.007 | |
| Chitosan, sample B | 0.097±0.004 | 0.008±0.001 | 0.185±0.006 | 0.034±0.002 | 0.250±0.005 | |
| Gauze bandage | 0.217±0.007 | 0.095±0.002 | 0.217±0.004 | 0.097±0.003 | 0.217±0.008 | |
The study of the water absorption capacity showed that the highest indicators of sorption could be seen in sample A – this exceeded similar indicators of samples B for distilled water by 2.92 ±0.13, 1.97±0.07 and 1.47±0.06 times at exposure of 15, 30 and 60 minutes, respectively, while for blood serum the same ratios were 12.87±0.59, 4.06±0.16 and 3.48±0.13 times at exposure of 15, 30 and 60 minutes respectively. The excess of the sorption capacity of sample A compared to the textile material (gauze bandage) was: for distilled water − 1.30 ± 0.06, 1.68±0.04 and 1.69± 0.03 times at exposure of 15, 30 and 60 minutes, respectively; for blood serum − 1.08± 0.03, 1.42±0.08 and 1.58±0.07 times at exposure 15, 30 and 60 minutes, respectively. Notable is that the textile material (gauze bandage) sorption capacity in relation to distilled water and blood serum featured no change over time, evidence to that being lack of any statistically significant differences along with increasing exposure time.
The laboratory data obtained through studying wound dressings and focusing on the level of polymers contained in blood plasma before the sorption and after it offered convincing proof to the fact that samples A had their sorption activity measured against high-molecular compounds exceeding this value in samples B and in the textile dressing.
The scanning electron microscopy results reveal that the outer layer of sample A is formed by chitosan of greater porosity (loose chitosan), while the inner layer contains a denser material. The outer shell, due to its high porosity and hygroscopic capacity, ensures higher adhesion of the sample with the surrounding tissues when the sample is introduced into the wound surface (Figure 4).
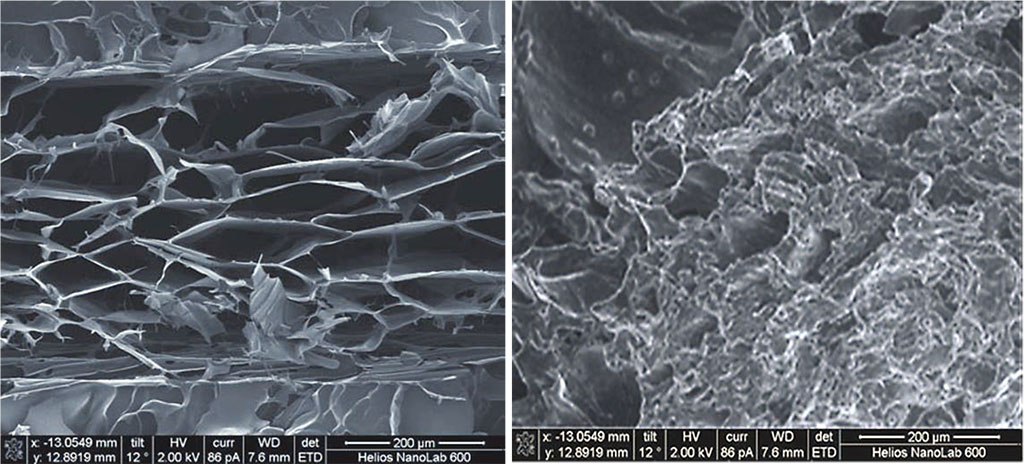
Figure 4. Scanning electron microscopy of sample A with directed (oriented) pores, magnification of ×350.
The scanning electron microscopy showed that sample B had an inverted structure, its outer layer containing a dense porous material, whereas the inner layer was found to be of higher porosity due to the structure modification (Figure 5).
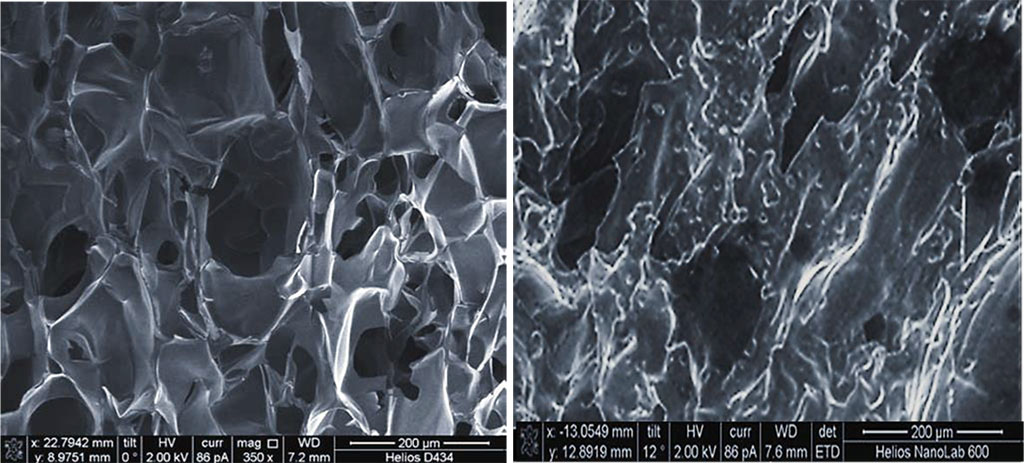
Figure 5. Scanning electron microscopy of sample B with non-directional (isotropic) pores, magnification of ×350.
Therefore, the comparative assessment of the sorption activity observed in chitosan-based wound dressings testifies that various advanced synthesis technologies allow creating medical application materials with predictable physical & chemical properties. This, in turn, can improve the effectiveness of treating wounds of various etiologies.
The research was carried out with the financial support of the Kuban Science Foundation in the framework of the scientific project No MFI-20.1-12/22 dated September 22, 2022.