- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Major research carried out in modern dentistry and maxillofacial surgery is commonly focused on long-term and stable results for implant rehabilitation of jaw defects and deformities in patients with bone and soft tissue losses at the site of planned implantation. Various osteoplastic materials, donor or the patient’s own bone, tissue-engineering and cellular products are common in clinical medicine, since guided regeneration can help not only recover the volume of bone tissue once lost, yet also ensure an outcome acceptable from both functional and aesthetic view. Guided bone regeneration employs methods of regenerative medicine and tissue engineering, with transplantation of native bone tissue and cell transplants, as well as various osteoplastic materials with osteoinductive and osteoconductive properties. In order to compare the structure of intact bone tissue obtained from intraoral and extraoral donor zones, histological and morphometric studies of bone autografts in 4 certified male cadavers with intact dentition were carried out. The results of histological studies in bone tissue autografts from intraoral and extraoral donor zones revealed that the highest density of bone rods could be observed in biopsies taken from the mandible outer oblique line area and chin symphysis. Whereas the lowest rate of the said factor we observed in the biopsies obtained from the iliac crest area. The highest rate of the inter-rod girder space was found in the area of the iliac crest, and the minimal – in biopsies from the parietal bone.
The highest bone vascularization level was identified at the iliac crest and the parietal bone, while its lowest levels were seen in biopsies taken from the area of the mandible outer oblique line. The density of cellular elements was found to be highest at the iliac crest. The lowest density level was registered in biopsies from the area of the mandible outer oblique line. The high rates of cellular elements in autografts from the iliac crest area can be accounted for by the predominance of spongy substance over cortical, while in bone biopsies taken from the mandible outer oblique line, chin symphysis, maxillary tuberosity and parietal bone, the share of cellular elements failed to exceed 27% within the total data set. This points at the predominance of cortical rather than spongy substance. In reconstructive bone plastic surgery for augmentation of the maxillary alveolar process and the mandible alveolar part with significant atrophy, it is reasonable to use autogenous transplants taken from intraoral donor zones. The autogenous transplants have cortical morphology and embryological origin similar to the jaw bones. Bone blocks from the donor zones of the mandible outer oblique line, the chin symphysis and the maxillary tuberosity have the highest density of the bone cortical substance and the duly sufficient amount of spongy substance.
Keywords: dental implants, histological examination, histomorphometric examination, intraoral donor zones, extraoral donor zones, autologous bone graft, maxillofacial surgery.
Restoration of the lost volume of bone tissue, as well as the replacement of bone defects and cavities when treating bone pathology (fractures, delayed consolidation, cystic rearrangement, tumors, false joints, infectious and post-infectious alterations, jaw deformities following surgeries), still remains one of the most relevant issues faced by modern medicine [1-4].
Advances in cell biology, regenerative medicine and materials science have turned intraosseous dental implantation into a modern high-tech field in both dentistry and maxillofacial surgery, which has attracted various experts to unite their efforts thus aiming at solving issues related to comprehensive rehabilitation of patients with serious – from the functional point of view – defects and deformities of the face bones, as well as various adentias. A decrease in the alveolar ridge mass caused by progressive bone atrophy affects possible intraosseous implantation due to a high risk of damage that may be caused to the mandibular nerve, the fundus perforation, and a ruptured maxillary sinus mucosa [5-9].
The high effectiveness of dental implantation, which achieves the most physiological indicators of adjustment and load distribution in the dental system, allows wider applications of prosthetics based on dental implants, ensuring proper functional and aesthetic properties of fixed dentures, and improve the life quality for patients suffering from partial or complete adentia [10-14].
As far as a long-term and stable result of dental implantation are concerned, important factors here are not only the sufficient volume and high quality of bone tissue, but also the keratinized gum attached at the intended implantation [15-17].
The use of histological and histomorphometric studies for analyzing bone microarchitectonics, allows the most reliable assessment of bones, both in view of its morphological features and qualitative parameters. It also helps identify the degree of the observable changes, and check the effectiveness of therapy and what effect it has on remodeling [18-25].
The informative value of common X-ray and dental techniques relies on obtaining a single-projection image of the respective jaw areas. To obtain a reliable image of the alveolar process during dental implantation, multi-projection cone-beam computed tomography (CBCT) is employed. CBCT has obvious advantages such as high resolution, a low radiation stress, and possible computer modeling of jaw bones volumetric reconstruction in view of the tissue density up to the trabecular package [26-35].
In case of patients with partial or complete adentia along with significant bone atrophy (the remainder of the bone tissue measuring at 1.0-3.0 mm in width), the most acceptable solution to restore the bone tissue volume of the alveolar process in height and width is bone grafting performed using the patient’s own bone. Autografts are considered the gold standard for restoring jaw bone tissue since they can offer the following: full biological compatibility; structural compliance; no immune response; potential for rapid revascularization with while developing organotypic bone tissue; progenitor cells present in transplants; biological safety since there is no risk of iatrogenic transmission of infection and potential antigenic reactions. Avascular bone autografts possess osteogenic, osteoconductive and osteoinductive properties, which translates into significantly improved remodeling and regeneration of bone tissue. The volume of resorption accompanied by bone fragment transplantation from one site to another depends on the size and structure of the bone graft, the bone morphology of the implant site, the biomechanical properties and fixation stability. The autograft revascularization is linked not to the nature of the donor zone alone, yet also to the regenerative potential of the recipient site. A well-vascularized bed and the decortication of not the graft only, but also of the implant site, will increase significantly the chances for successful engraftment [36-38].
Researchers offer reliable proof to the fact that following the transplantation to the recipient area, bone structures, viable osteoblasts, collagen and non-collagen extracellular matrix proteins, minerals and morphogenetic bone proteins still remain in autografts [39].
While working with bone autografts, we could observe the following: avoiding bone overheating when taking a bone block to reduce its injury and to preserve as many of viable cells as possible; reducing the time between graft collection and its transfer to the recipient area; the need to store the bone block in a moist and cool environment; the need to overlap bone blocks and the area being reconstruction with a resorbed membrane thus aiming to avoid excessive resorption [40-41].
Depending on the extent and degree of jaw alveolar process bone atrophy, clinicians employ bone grafts coming from various sampling zones. In order to eliminate bone defects with a length of up to 4 teeth, bone blocks from intraoral donor zones are used: the outer oblique line and the anterior edge of the mandible branch, the chin symphysis, the coronal process and the maxillary tuberosity. Greater defects are eliminated by bone blocks taken from extraoral donor zones: the iliac crest, the cranial vault bones, the anterior tibia, the fibula, and the rib. The bone sampling zone is to be selected in view of the proposed reconstruction volume, the bone grafting methods to be employed, and the patient’s preference. Bone autografts are used in shapes of bone blocks, bone chips or a combination of the two [42-43].
Due to their embryological similarity with the jaw tissues, autografts from intraoral donor zones have a lesser degree of resorption, faster regenerate and revascularize in the recipient zone, unlike extraoral zones [44].
Intraoral bone autografts offer the following advantages: good surgical access; proximity of donor and recipient sites; shorter surgical intervention and anesthetic assistance required; lack of sutures and postoperative scars remaining on the skin; morphological specifics of the donor and the recipient sites; potential for bone plastic surgery in an outpatient facility also using local anesthesia; the lowest resorption of intraoral bone autografts if compared with extraoral areas. Extraoral bone sampling zones can be justified in cases where the volume of the bone tissue obtained from intraoral areas is insufficient or there is no way to perform surgical sampling in the oral cavity [45].
The mandible outer oblique line, the anterior mandible process or chin symphysis are known to have a potential for offering a 5-10 cm3 graft, whereas another up to 2 cm3 can be obtained from the coronal process or the maxillary tuberosity area. The advantages of obtaining a bone autograft from the chin symphysis area include the largest possible volume of bone material (compared with other intraoral zones); lower pain at the intervention zone through the postoperative period; a short rehabilitation period, and lack of scars left on the skin. However, there are also disadvantages, namely, damaged roots of the teeth central group, during graft collection, and chin and lower lip skin paresthesia in case of damage to the mental nerve. As for the advantages of obtaining bone tissue from the retromolar area, these include the lowest number of complications affecting the donor area (inflammations, scars, suture divergence), and no impaired sensitivity of the mucous membrane vestibular surface and the lip skin sensitivity, while autografts in this area feature an insignificant rate of resorption [46].
Extraoral donor zones allow obtaining larger autografts (iliac crest − 50-120 cm3, cranial vault and tibia – 20-40 cm3). However, an extra surgical field will serve a source of serious discomfort for the patient at the recovery stage. The advantage of obtaining autografts from the iliac crest is that they can be used as large bone blocks for simultaneous reconstruction, as well as bone chips can be obtained for sinus lifting, while viable osteoblasts remain for up to 3 hours, and revascularization starts 48 hours following transplantation. The disadvantages of the iliac crest as a donor zone – given the large amount of spongy substance – include low mechanical strength and low resistance to bone graft resorption after transplantation; disturbed attachment of the wide fascia of the femur; possible damage to the n. cutaneous femoralis lateralis; cosmetic issues; ruptured sacroiliac joint; fractured iliac wing or its anterior spine; risk of inguinal hernia; neurovascular injuries; infection and deep hematomas.
Indications for a graft taken from the proximal tibia include sinus lifting surgeries, augmentation by splitting, as well as filling larger cavities after cystectomy, while the disadvantages of this donor zone include potential damage to anatomical structures with possible pathological fracture, a postoperative scar remaining in an area that is of aesthetic value, and damaged knee ligaments. The common embryological origin shared with the jaws, provides autografts from the parietal bone with such features as high mechanical strength and low resorption, these making such grafts good for bone grafting using blocks. Due to the low thickness, though, there is a high risk of fracture at the modeling stage. The complications affecting the donor zone are possible damage to the dura mater during graft collection, neurological complications, postoperative scars, and alopecia along the incision line [47]. Since it is impossible to install dental implants in patients with secondary adentia and severe atrophy of the alveolar process (part) of the jaws with no prior surgical preparation, an in-depth study of intraoral and extraoral donor zones appears to be a reasonable option in order to expand the clinical indications.
Aim: to carry out comparative evaluation of the intact bone tissue obtained from intraoral and extraoral donor zones based on histological and morphometric studies of bone autografts.
The material for the study was obtained through a sectional study of 4 certified male bodies with intact dentition, carried out in the thanatology department of the Forensic Medical Examination Bureau. The age of the corpses was identified as the II adulthood period (36-60). The histological and morphometric studies of bone tissue preparations were performed on the premises of the Consulting Clinical Bureau for Postmortem Examination. The inclusion criteria were: no trauma-related injuries of bone biopsies at the examined donor zones; no signs of pathological changes or diseases (connective tissue diseases, endocrine pathologies; significant signs of chronic exogenous intoxication). Bone grafts that were used to eliminate defects in the maxillary alveolar process and the mandibular alveolar part were taken from intraoral and extraoral donor zones.
The intraoral donor zones were bone blocks from the area of the mandible outer oblique line, the chin symphysis and the maxillary tuberosity. As far as extraoral sources of bone tissue are concerned, then bone blocks from the parietal bone and iliac crest were examined. The size of the bone biopsies taken from the donor zones was 5×5 mm. The obtained material was fixed in a 10% neutral buffered formalin solution for 48 hours. The fixation completed, decalcification was performed in a 25% Trilon B solution of organic acid, followed by dehydration in isopropanol of ascending concentration employing a Leica TR1020 (Leica Microsystems, Nussloch, GmbH, Germany) automatic tissue carousel-type processor for histological tissue treatment (Fig. 1), with the material further covered with paraffin (Histomix, Biovitrum, Russia), using a specific pouring system.

Figure 1. Leica ТР1020, automatic system for tissue histological treatment
Once the processing and paraffin pouring was over, serial sections (5-7 microns) were prepared using a Leica RM2235 manual rotary microtome (Leica Microsystems, Germany) (Fig. 2).

Figure 2. Leica RM2235, manual rotary microtome.
The paraffin sections were stained with hematoxylin and eosin (Biovitrum, Russia) and picrofuxin, following Van Gieson’s method using the Raffaello staining unit (DIAPATH, S.p.A., Italy). Microscopic examinations were carried out with a Leica DM2500direct light optical microscope (Leica Microsystems), at magnifications of ×100, ×200, ×400, ×800 (Fig. 3).
Figure 3. Leica DM2500, optical microscope
Morphometric measurements for quantitative characterization of bone biopsies from intraoral and extraoral donor zones were performed in the ImageJ software (National Institutes of Health, USA) on a Leica DM2500 set. At a magnification of ×200, the following parameters were evaluated on each histological section in six fields within view on an image of 100×75 microns (area: 7.5 mm2): the bone rod area; the inter-rod area; the vascularization degree; the number of cells. A magnification of ×400 on each histological section within view on an image of 15×12.5 microns (area: 0.18 mm2) was used to identify the following: the number of osteocytes within the view field; the number of osteoblasts within view; the number of osteoclasts within view. The statistical data processing was done using the nonparametric Mann-Whitney criterion on the Microsoft Windows 10.0 operating system in the Microsoft Excel 2019, Statistica 12.0 software package (StatSoft Inc., USA).
Figure 4 contains the results of histological examination of the bone biopsy obtained at the chin symphysis (Symphysis mandibulae).
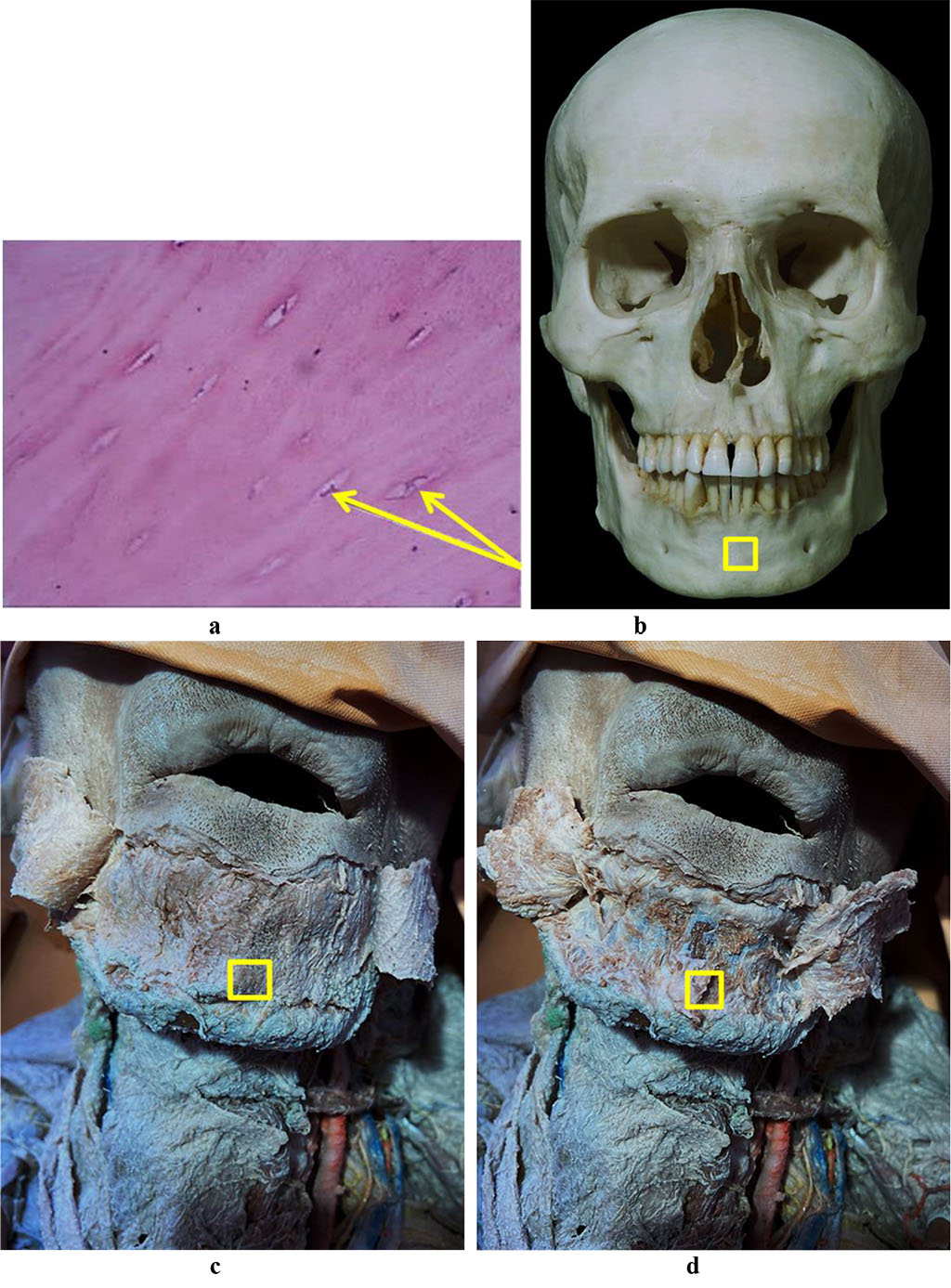
Figure 4. Bone biopsy taken from the chin symphysis area: a – a histological preparation of intact bone from the chin symphysis area (×400, hematoxylin-eosin staining; osteocytes indicated by arrows); b – a section of the mandible donor zone to obtain a chin autograft; c – an anatomical preparation after the skin flap detachment; d – an anatomical preparation after the muco-periosteal flap detachment.
Tables 1-2 offer the results of histological and morphometric parameters of a bone biopsy obtained from the chin symphysis area.
Table 1. Histological parameters of the intact bone tissue biopsy taken from the chin symphysis area (share of the area as per each parameter within the six view fields), (M±m), (%)
| Histological parameters | Parameter share in entire data set |
| Bone rods | 81.26 ± 5.43 |
| Inter-rod space | 3.19 ± 1.82 |
| Vascularization degree | 0.94 ± 0.17 |
| Cell elements | 14.61 ± 1.15 |
Table 2. Morphometric cytological parameters of the intact bone tissue biopsy from the chin symphysis area (share of the area as per each parameter within the view field), (M±m), (%)
| Morphometric cytological parameters | Parameter share in entire data set |
| Osteocytes | 92.97 ± 3.64 |
| Osteoblasts | 7.03 ± 2.19 |
| Osteoclasts | 0.00 |
Figure 5 reveals the results of the histological examination of a bone biopsy taken from the mandible outer oblique line the area (Linea obliqua).
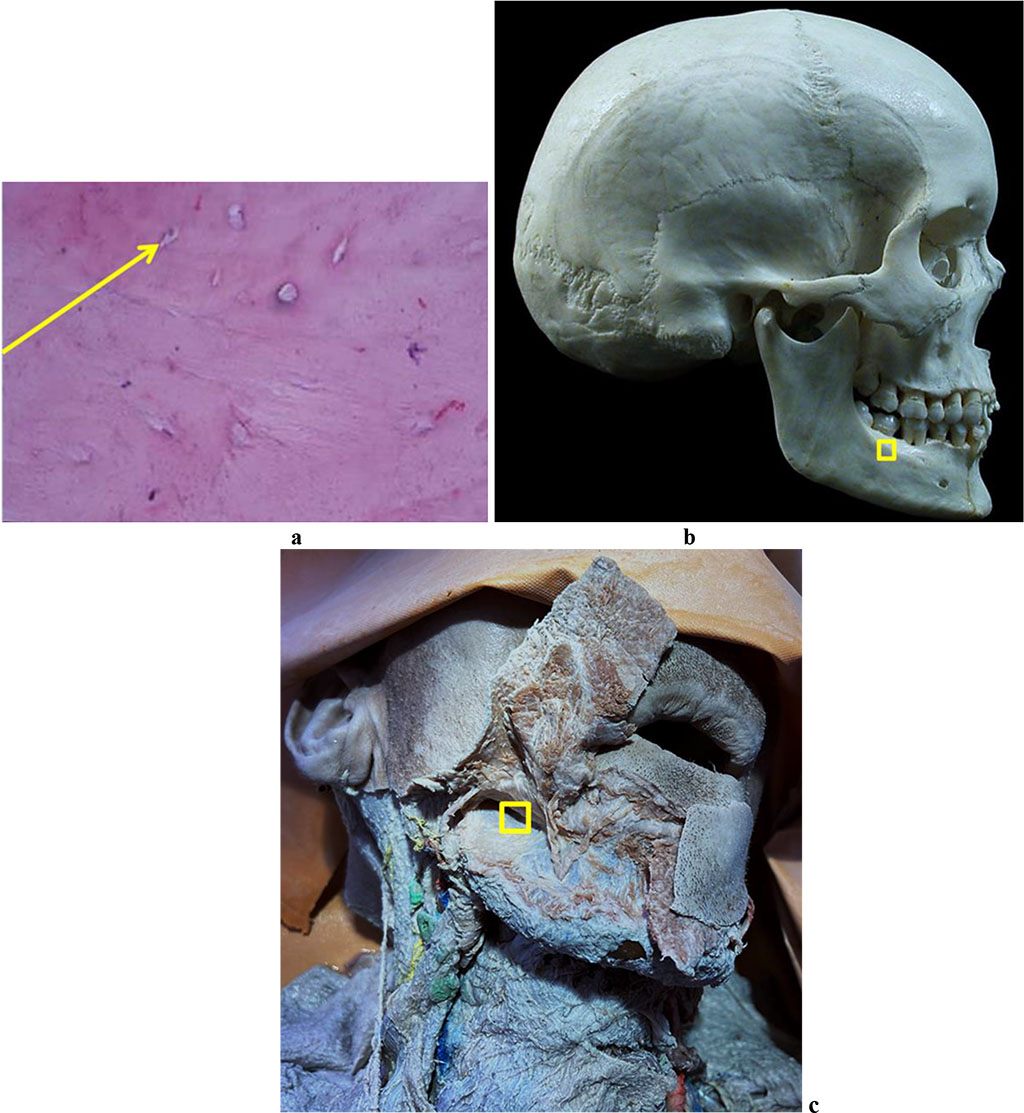
Figure 5. Bone biopsy from the mandible outer oblique line area: a – a histological preparation of intact bone from the mandible outer oblique line area (×400, hematoxylin-eosin stain, osteocyte indicated by the arrow); b – a section of the donor zone for obtaining an autograft from the mandible outer oblique line area; c – an anatomical preparation after the muco-periosteal flap detachment.
The histological and morphometric measurements obtained through studying the bone biopsy taken from the mandible outer oblique line area are to be seen in Tables 3-4.
Table 3. Histological parameters of the intact bone tissue biopsy taken from the mandible outer oblique line area (share of the area as per each parameter within the six view fields), (M±m), (%)
| Histological parameters | Parameter share in entire data set |
| Bone rods | 90.47 ± 2.16 |
| Inter-rod space | 4.08 ± 1.63 |
| Vascularization degree | 0.07 ± 0.01 |
| Cell elements | 5.38 ± 0.72 |
Table 4. Morphometric cytological parameters of the intact bone tissue biopsy taken from the mandible outer oblique line area (share of the area as per each parameter within the view field), (M±m), (%)
| Morphometric cytological parameters | Parameter share in entire data set |
| Osteocytes | 95.64 ± 3.03 |
| Osteoblasts | 4.36 ± 1.48 |
| Osteoclasts | 0.00 |
Figure 6 offers a view at the results of a histological study of the bone biopsy obtained from the maxillary tuberosity (Tuber maxillae) (Fig. 6).
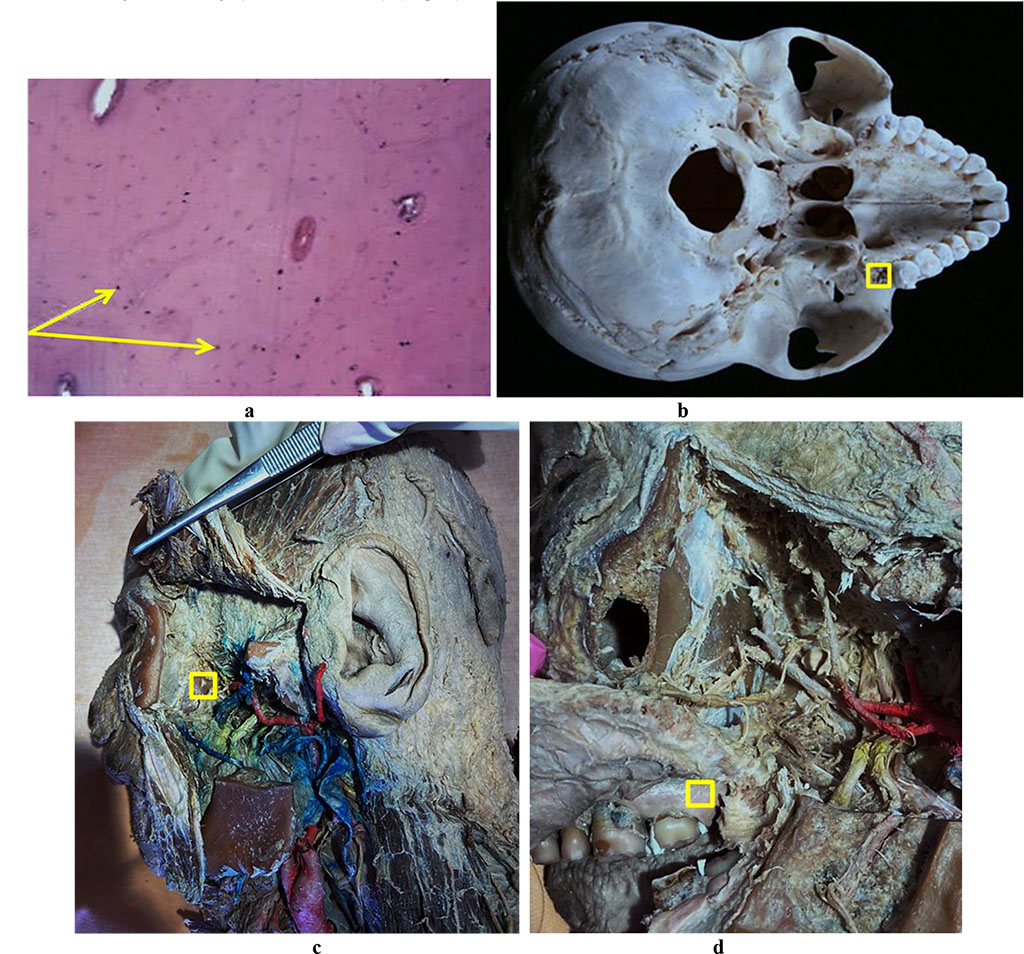
Figure 6. Bone biopsy obtained from the maxillary tuberosity area: a – histological preparation of intact bone taken from the maxillary tuberosity area (×400, hematoxylin-eosin staining, osteocytes indicated by an arrow); b – a donor zone section in order to obtain an autograft from the maxillary tuberosity area; c – an anatomical preparation after the fasciocutaneous flap detachment; d – an anatomical preparation after the muco-periosteal flap detachment.
Tables 5-6 contain the results of histological and morphometric data of the bone biopsy taken from the maxillary tuberosity area.
Table 5. Histological parameters of the intact bone tissue biopsy from the maxillary tuberosity area (share of the area as per each parameter within the six view fields), (M±m), (%)
| Histological parameters | Parameter share in entire data set |
| Bone rods | 66.08 ± 7.96 |
| Inter-rod space | 6.27 ± 2.09 |
| Vascularization degree | 1.46 ± 0.22 |
| Cell elements | 26.19 ± 0.48 |
Table 6. Morphometric cytological parameters of the intact bone tissue biopsy of from the maxillary tuberosity area (share of the area as per each parameter within the view field), (M±m), (%)
| Morphometric cytological parameters | Parameter share in entire data set |
| Osteocytes | 91.06 ± 1.76 |
| Osteoblasts | 8.94 ± 2.19 |
| Osteoclasts | 0.00 |
Figure 7 offers the results of histological examination of the bone biopsy taken from the parietal bone (Os parietale).
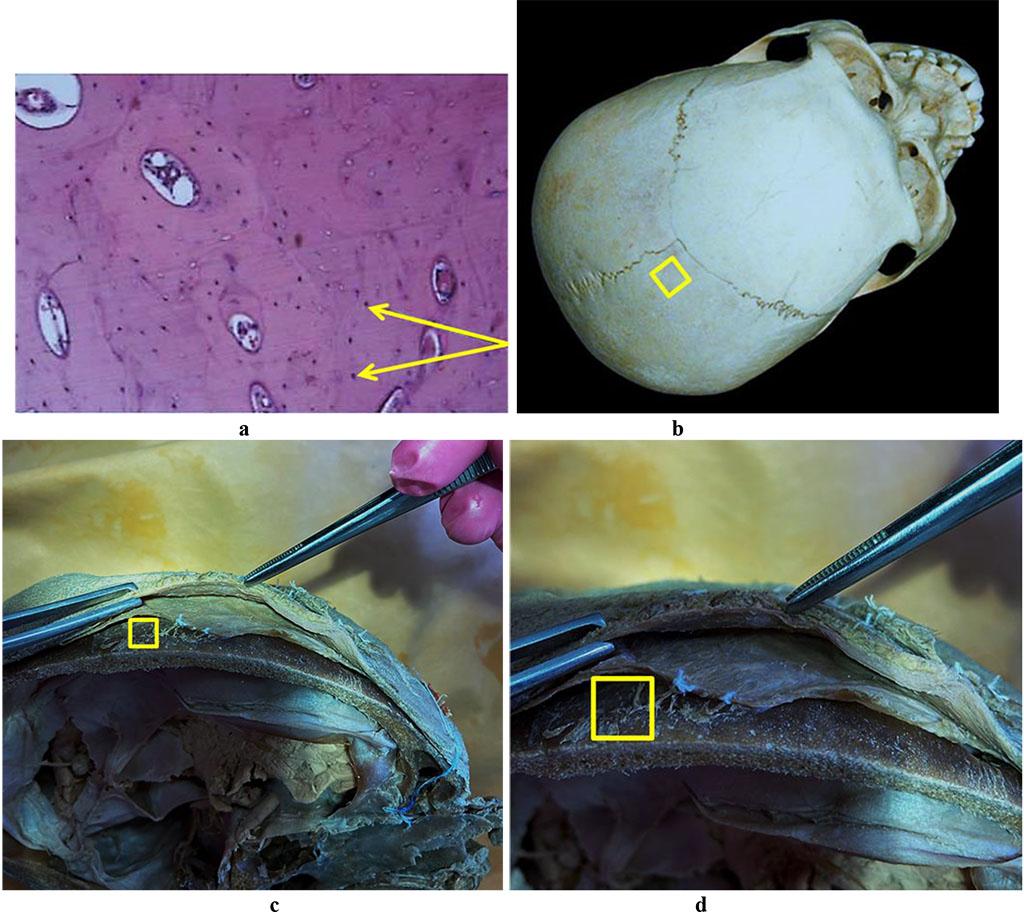
Figure 7. Bone biopsy from the parietal area: a – histological preparation of intact bone from the parietal area (×400, hematoxylin-eosin staining, osteocytes indicated by an arrow); b – a section of the donor zone done to obtain an autograft from the parietal area; c, d – an anatomical preparation after the fasciocutaneous flap detachment.
The histological and morphometric outcomes of the bone biopsy obtained from the parietal bone can be seen in Tables 7-8.
Table 7. Histological parameters of the intact bone biopsy taken from the parietal bone area (share of the area as per each parameter within the six view fields), (M±m), (%)
| Histological parameters | Parameter share in entire data set |
| Bone rods | 71.37 ± 6.88 |
| Inter-rod space | 0.16 ± 0.03 |
| Vascularization degree | 3.61 ± 0.74 |
| Cell elements | 24.86 ± 0.59 |
Table 8. Morphometric cytological parameters of the intact bone biopsy obtained from the parietal bone area (share of the area as per each parameter within the view field), (M±m), (%)
| Morphometric cytological parameters | Parameter share in entire data set |
| Osteocytes | 98.43 ± 0.52 |
| Osteoblasts | 1.49 ± 0.16 |
| Osteoclasts | 0.08 ± 0.03 |
The results of histological examination of the bone biopsy from the iliac crest area (Crista iliaca) are to be seen in Fig. 8.
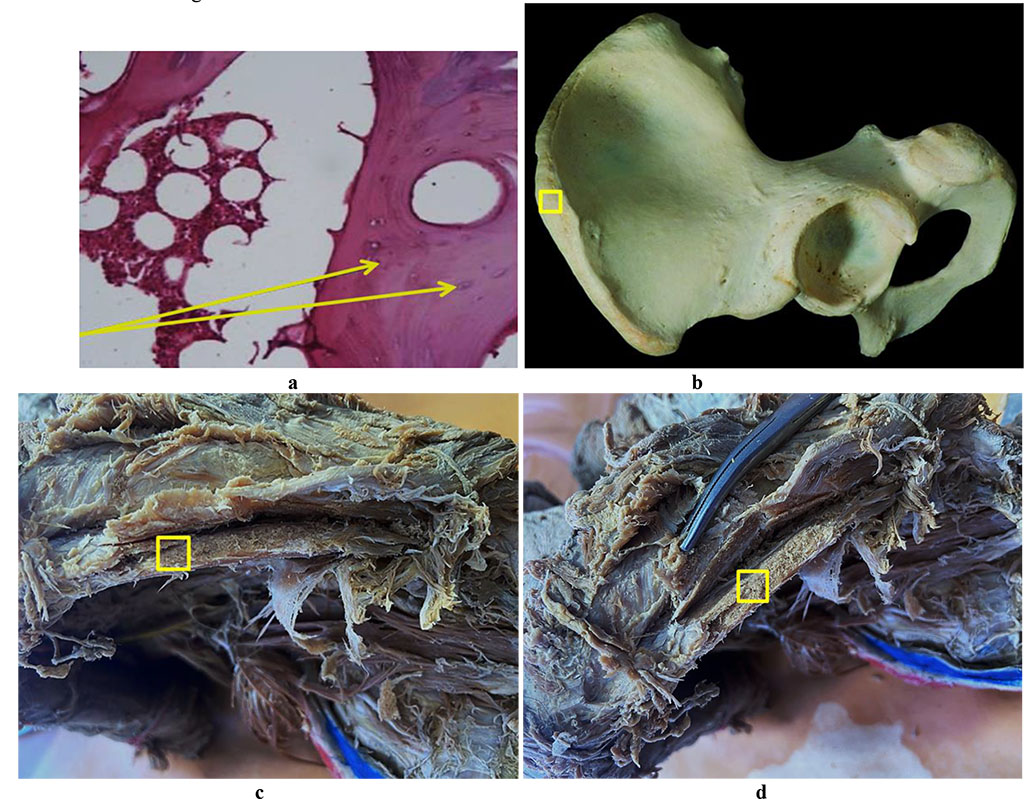
Figure 8. Bone biopsy from the iliac crest area: a – histological preparation of intact bone from the iliac crest area (×400, hematoxylin-eosin staining, osteocytes indicated by an arrow); b – a donor zone section to take an autograft from the iliac crest area; c, d – an anatomical preparation after the fasciocutaneous flap detachment.
The histological and morphometric results of the iliac crest area bone biopsy can be seen in Tables 9-10.
Table 9. Histological data for the biopsy of intact bone tissue obtained from the iliac crest area (share of the area as per each parameter within the six view fields), (M±m), (%)
| Histological parameters | Parameter share in entire data set |
| Bone rods | 25.24 ± 1.93 |
| Inter-rod space | 18.42 ± 4.61 |
| Vascularization degree | 4.13 ± 0.86 |
| Cell elements | 52.21 ± 6.37 |
Table 10. Morphometric cytological parameters of the intact bone biopsy from the iliac crest area (share of the area as per each parameter within the view field), (M±m), (%)
| Morphometric cytological parameters | Parameter share in entire data set |
| Osteocytes | 96.73 ± 1.82 |
| Osteoblasts | 3.27 ± 0.29 |
| Osteoclasts | 0.00 |
The histological examination of the bone biopsies taken from the chin symphysis area and from the area of the mandible outer oblique line has revealed that the bone tissue features a well-defined cortical plate and spongy bone. The inter-rod space is filled with bone marrow reticular stroma with no foci of hematopoiesis and with areas of rough-fiber based poorly shaped connective tissue. The cortical bone mass is of a lamellar structure with well-shaped Haversian canals. Osteons are short, based along the bone surface. Part of the trabeculae includes an inhomogeneous bone matrix, where the trabeculae internal structure features a bone matrix with empty lacunae, as well as with clefts and stratified areas of bone matter. The surface of the rods contains bone tissue with osteocytes, with areas of immature bone tissue occurring sometimes. The inner surface of the Haversian canals and the outer surface of the trabecular part feature signs of smooth resorption, which reveal themselves as splitting bone substance. The inter-rod space is filled with loose fibrous unshaped connective tissue. The trabeculae of the autograft spongy bone have a horizontal orientation, with a mature structure with individual osteoids and proliferating osteoblasts with no osteoclasts. In most cases, the Haversian canals do not contain vessels. As for the chin symphysis area, the spongy layer is basically not to be seen, and the bone tissue appears as a compact layer of homogeneous, dense bone, while in the outer oblique line area, the spongy layer was observed to have a rough-grained pattern. Osteocytes located among the fibrous bone matrix are of a stellate shape, their nuclei located in the central part of the cells (Fig. 9).
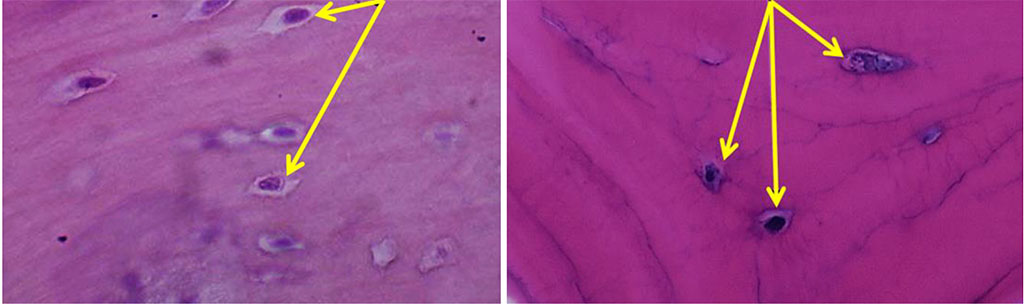
Figure 9. Osteocytes on histological preparations of the intact bone from the chin symphysis area (a) and from the outer oblique line area (b); (×800, hematoxylin-eosin stain).
Osteocytes from the chin symphysis area are oriented along the bone plate course, whereas osteocytes from the outer oblique line area – along the circles around the Haversian canals (Fig. 10).
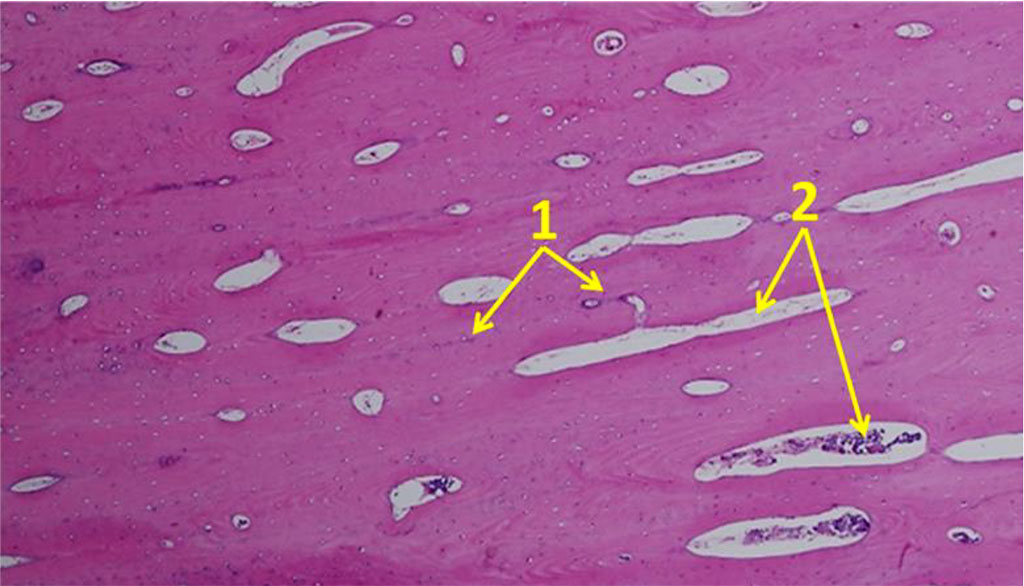
Figure 10. Orientation of osteocytes in relation to the Haversian canals on histological preparations of the intact bone from the chin symphysis area (×100; hematoxylin-eosin staining; 1– osteocytes; 2 – Haversian canals).
The histological structure of the bone biopsy obtained from the maxillary tuberosity area differed from what could be observed in the biopsies taken from the chin symphysis area and of the mandible outer oblique line, the difference being obvious through the vertical trabeculae arrangement, the fine-meshed structure of the spongy substance, and the orientation of the bone plates along the concentric circles around the Haversian canals (Fig. 11).
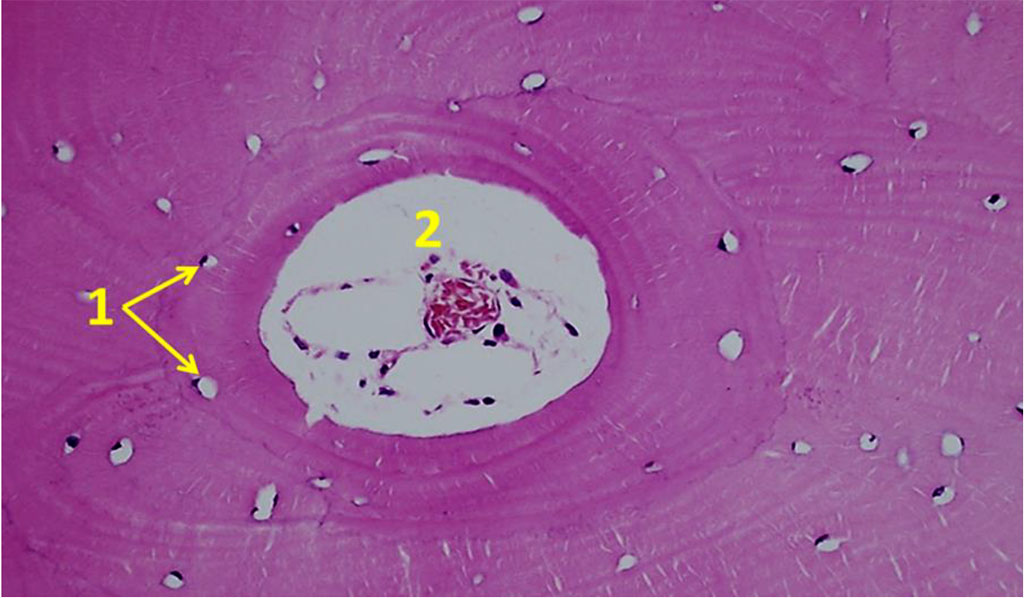
Figure 11. Orientation of osteocytes in relation to the Haversian canals on histological preparations of the intact bone from the maxillary tuberosity area (×100; hematoxylin-eosin stain; 1– osteocytes; 2 – Haversian canal).
The histological study of the bone biopsies obtained from the iliac crest area suggest that the cortical plate has a common average thickness, while the spongy bone trabeculae are large and wide. The intertrabecular space is filled with red bone marrow, sometimes featuring signs of being replaced by yellow marrow. The cortical plate has large Haversian canals lined with endosteum, which contains bone marrow reticular stroma, passing into yellow marrow. The spongy bone trabeculae are also covered with endosteum, with no signs of osteoclastic resorption, while osteoblastic activity is low.
The data obtained through the histological examination of bone biopsies taken from the parietal bone area indicate that the bone tissue appears as a loose cortical plate with a layer of spongy bone underneath. The cortical plate is narrow, eroded by Volkmann's canaliculi. The spongy bone contains a combination of narrow bone trabeculae and trabeculae of conventional thickness. Bone trabeculae contain a small number of areas with incomplete bone tissue mineralization. The bone trabeculae surface has round or cubic-shaped osteoblasts with basophilic nuclei surrounded by an osteoid. Bone trabeculae are mostly lined with flattened cells, which are osteoblast precursors. There are isolated foci of osteoclastic activity to be seen occasionally, which came along with smooth resorption of the bone-rod surface. The inter-rod space is filled with a soft fibrous reticular connective tissue, moderately vascularized. No adipose or circulatory tissues were observed in the reticular stroma.