- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2025. 15; 1. DOI 10.35630/2025/15/1.105
Adopting the endomicrosurgical methods for treating diseases of the nose and paranasal sinuses into the practice of otolaryngologists, and the methods of dental implantation and sinus lifting into the practice of dentists, has become an incentive for a more in-depth study of the structural organization of the sinus membrane (Schneiderian membrane) and the processes of its post-traumatic regeneration.
The Aim of the Study is to investigate the structure of the maxillary sinus membrane and to establish the presence of the cells capable of osteogenic differentiation.
Material and Methods: The biological material was obtained from 20 men aged 38 to 56 years who underwent surgery due to iatrogenic sinus perforation and removal of foreign bodies. A general idea of the structure of the sinus membrane was formed based on histological sections stained by using hematoxylin and eosin; cells-areas of proliferative processes were determined immunohistochemically by the markers ki-67 and a-SMA.
Results: The membrane of the maxillary sinus is characterized by a layered arrangement of the structures of the mucosa and periosteum, which are in close structural and functional connection. It is in them and the endothelium of the vessels of the lamina propria of the mucosa that cells with proliferative activity are determined. In the lamina propria of the mucosa, a-SMA cells are present. The periosteum is represented by a single bundle of collagen fibers and does not have a pronounced cambial layer, and accordingly has a low regenerative potential.
Conclusion: The sources of osteoprogenitor cells may be endothelial and connective tissue cells that express ki-67 and cells expressing a-SMA. Given the functional significance of a-SMA positive cells, it can be assumed that in the sinus membrane they can: regulate cyclic changes in the degree of swelling of the mucosa; play a directional role in the migration of the epithelial layer during sinus pneumatization and participate in the directed differentiation of cells, including osteoprogenitor cells.
Keywords: maxillary sinus, Schneiderian membrane, mucosa, periosteum, periosteal coverage, a-smooth muscle actin.
Schneiderian membrane is a special lining of the inner surface of the maxillary sinus (MS). It is known that it consists of a mucosa that has two layers in its structure: a layer of multi-row ciliated epithelium and a layer of loose fibrous connective tissue underlying the epithelium. A feature of this membrane is the presence of not only a mucosa, but also a periosteum [1]. Often, in the practice of otolaryngologists, this entire complex is called mucoperiosteum [2].
Recently, the stimulus for in-depth study of the structural organization of the Schneiderian membrane has become: on the one hand, the introduction of endomicrosurgical methods for treating diseases of the nose and paranasal sinuses, which involve partial or complete removal of the mucosa lining and which require monitoring of post-traumatic regeneration processes [3, 4]; on the other hand, the widespread introduction of dental implantation methods and sinus lifting into dental practice [5]. As it turned out, the performance of the described surgical procedures in 30% of cases can be accompanied by perforation of the sinus membrane [6]. The main causes and predisposing factors for the occurrence of this pathological condition can be: excessive pressure on the membrane [7], a decrease in its elasticity or a change in thickness [8], the presence of additional partitions in the sinus [9]. However, regardless of the cause of the damage, it is important for otolaryngologists to understand the essence of all reactive processes that can unfold in a given area of the organ and the cells that can be involved in the regeneration processes.
It is known that the mucosa of the airways has sufficient regenerative potential. On the part of the epithelial layer of the mucosa, regeneration is achieved thanks to basal cells. Of no small importance in the regeneration of the mucosa are the cells of the underlying connective tissue and the extensive vascular network located in the lamina propria of the mucosa. As for the periosteum of the Schneiderian membrane itself, it is only known that its regenerative potential is significantly lower [10]. This is evidenced by the slow and continuous decrease in the volume of the bone tissue of the maxilla and, conversely, an increase in the volume of its air cavity.
Based on the mentioned above, the aim of the study was to examine the histostructural organization of the Schneiderian membrane by the method of classical light microscopy and immunohistochemistry using the ki-67 proliferation marker and the a-SMA marker.
The study was conducted at Outpatient Center No. 1, Samara (license No. LO-63-01-004326 dated August 10, 2017 (issued for an indefinite period of time)). The object of the study was the Schneiderian membrane of the anterior wall of the maxillary sinus. The material for the study was obtained from 20 men aged 38 to 56 years who underwent surgery due to iatrogenic sinus perforation and removal of foreign bodies from the maxillary sinus.
The maxillary sinus was accessed through an incision in the mucosa of the oral vestibule at the level of the 4th to 5th upper teeth along the transitional fold and then through the anterior wall of the maxilla. A fragment of the inner lining of the sinus with an area of 6 mm was taken with a bone raspatory at the site of the trepanation hole.
The biological material was fixed in 10% buffered formalin with subsequent hardware processing (Leica ASP 300, Germany). The dehydrated material was embedded in paraffin, and sections were prepared with the thickness of 4 μm.
To obtain the general idea of the structure of the Schneiderian membrane the prepared sections were stained by using hematoxylin and eosin. To identify the cells with proliferative activity and the cells capable of expressing smooth muscle actin (a-SMA) in the structure of the Schneider's membrane, an immunohistochemical study was performed using a monoclonal antibody (Dako, Denmark). The study was conducted in accordance with the standard protocols of the Ultra Vision ONE visualization system. During the procedure, antigen determinants were unmasked (Dako, Denmark). The sections were counterstained by using hematoxylin and embedded in a glycerol-gelatin medium. The results were assessed after setting up positive and negative controls.
Light microscopy of the obtained Schneiderian membrane preparations showed that the general structure of the maxillary sinus mucosa is characterized by a regular layered arrangement of its constituent structures (Fig. 1). The standard was the covering multi-row ciliated epithelium localized on the basal membrane and the lamina propria of the mucosa formed by loose fibrous connective tissue. In the epithelium, there were areas of desquamation with exposure of the basal membrane. In 70% of cases, manifestations of focal or diffuse chronic productive inflammation were noted, more pronounced in the superficial layers of the mucosa. The periosteal coverage of the sinus membrane had a single-layer structure, into which fibers of the extracellular matrix from the lamina propria were woven in some areas (Fig. 1a). The fibers of the connective tissue in the periosteum layer formed a single bundle of fibers with a spatial orientation parallel to the surface of the epithelium. In all the studied objects, the inner cellular layer of the periosteum, characteristic of the classical periosteal coverage, was not visualized.

Fig. 1. Section of the Schneiderian membrane from the anterior wall of the maxillary sinus of a 49-year-old man. Staining by using hematoxylin and eosin. Magnification 100X. 1- epithelium; 2- loose connective tissue of the lamina propria; 3-periosteum.
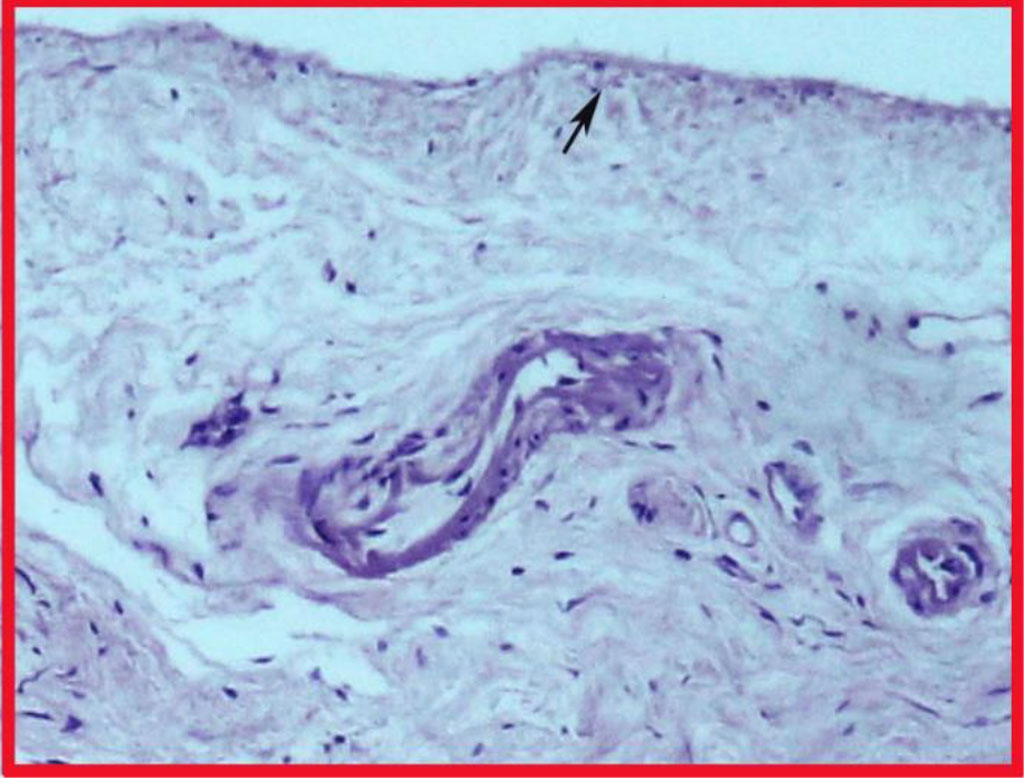
Fig. 1a. Highlighted area in Fig. 1. Fibers interwoven into the periosteum (highlighted by the arrow).
Taking into account the fact that bone tissue regeneration is still possible in the maxilla, we decided to clarify which cells in the mucoperiosteum exhibit the ability to proliferate. Detection of the Ki-67 antigen showed that low proliferative activity of cells is observed in the Schneiderian membrane. This specific nuclear protein was detected in the cells of the integumentary epithelium, in single cells of the connective tissue and the endothelium of the vessels of the lamina propria (Fig. 2, 2a). No Ki-67-positive cells were detected on the periosteum side.
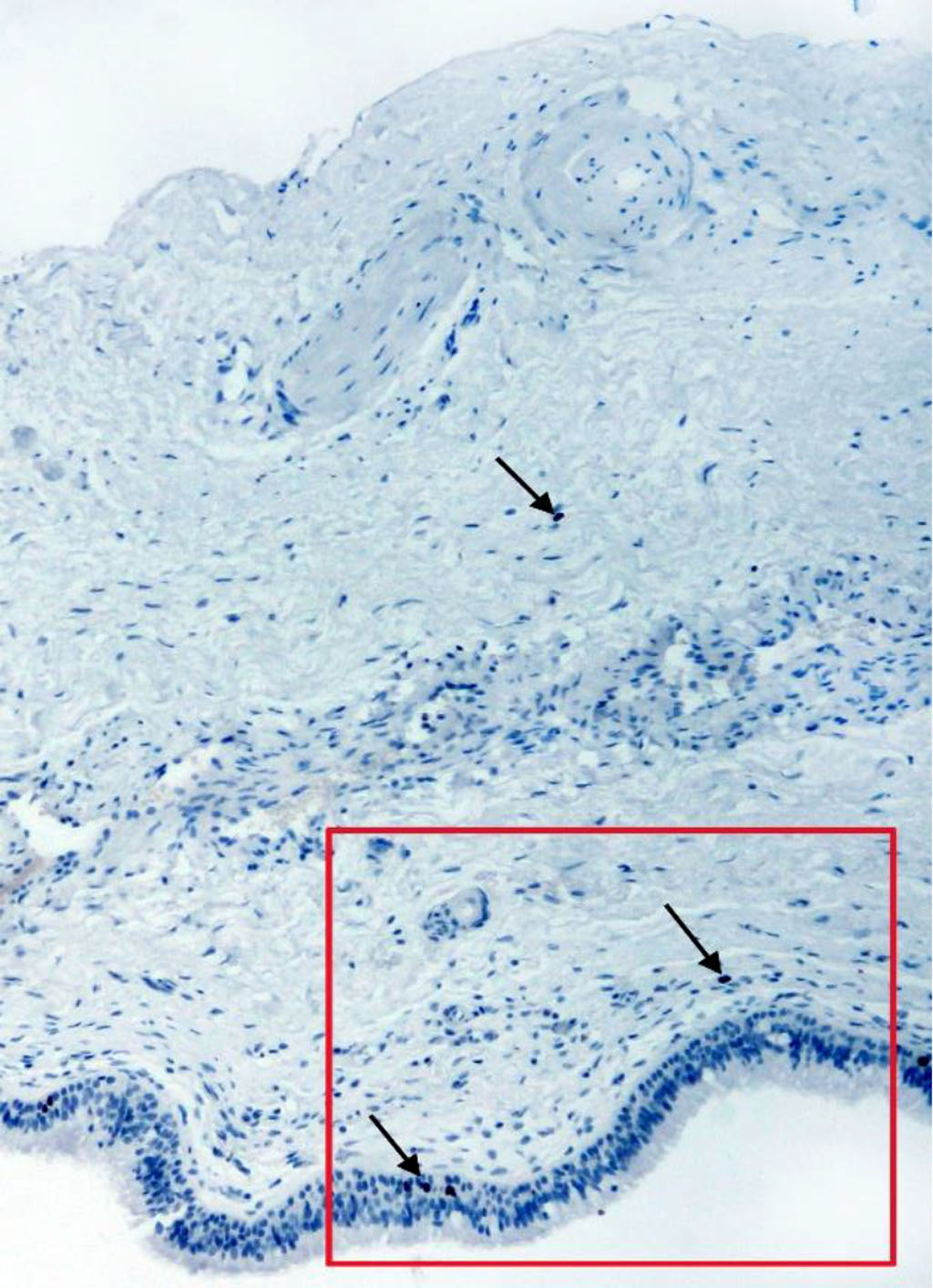
Fig. 2. Section of the membrane from the anterior wall of the maxillary sinus of a 46-year-old man. IHC staining for the Ki-67 antigen. Magnification 100X. Arrows indicate positive cells.
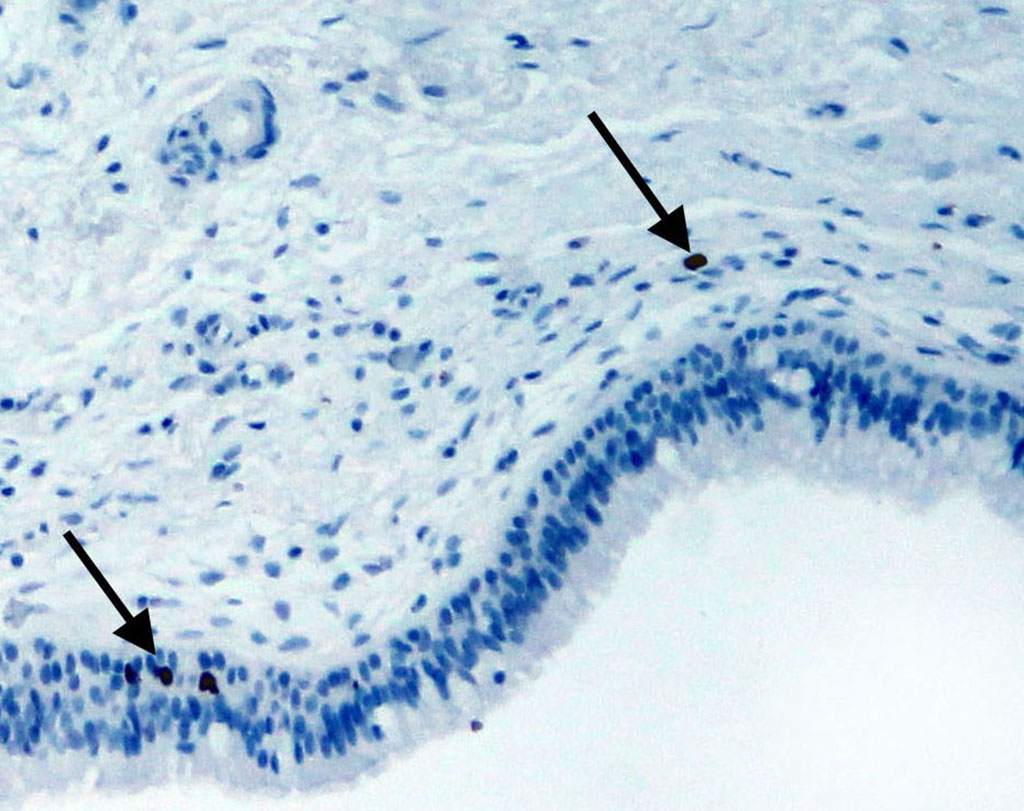
Fig. 2a. The highlighted area in Fig. 2.
Immunohistochemical examination of Schneiderian membrane cells for alpha-smooth muscle actin showed that single small positive cells were found in the periosteum (Fig. 3). At the same time, our method of examination enabled to identify muscular-type vessels (arteries and veins) and diffusely scattered elongated cells with cytoplasm staining for a-SMA in the structure of the lamina propria (Fig. 4).
Alpha-smooth muscle actin belongs to the actin family of proteins that form the microfilaments of the cell skeleton. It plays an important role in maintaining the cell structure and is responsible for performing mechanical functions, providing intracellular contractility and/or tension, as well as cellular motility [11].
Alpha-smooth muscle actin is predominantly expressed in smooth muscle cells, but it can also be present in some other cell types, such as pericytes, vascular endothelial cells [12], and activated fibroblast populations or myofibroblasts [13,14].
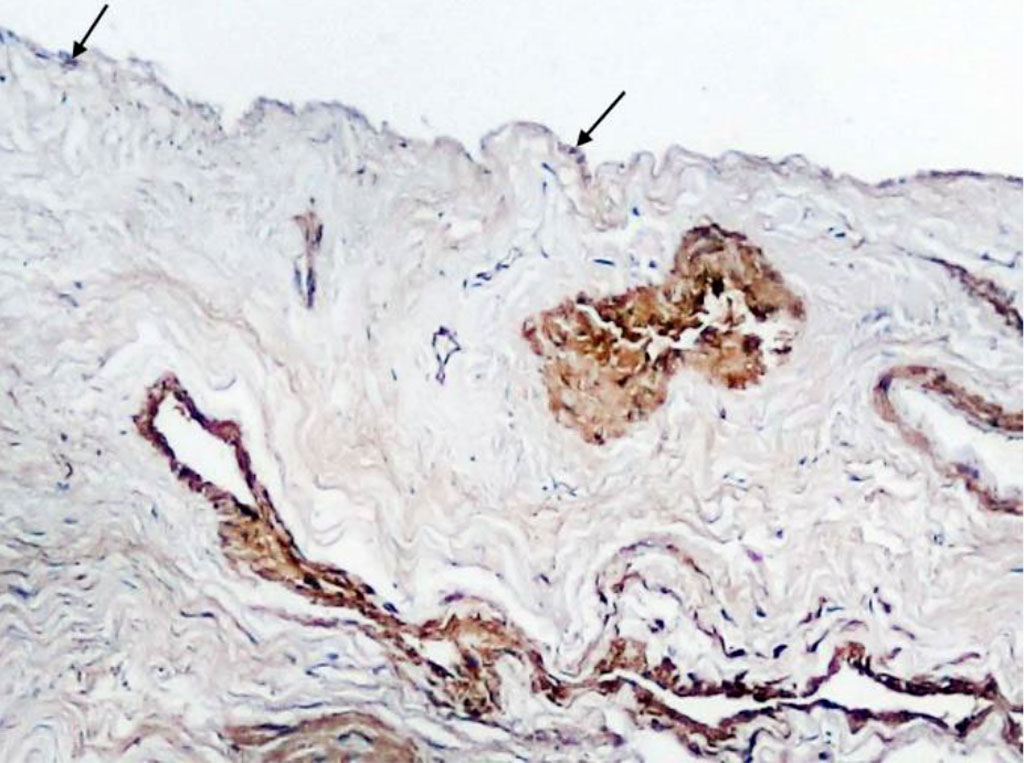
Fig. 3. Section of Schneiderian membrane from the anterior wall of the maxillary sinus of a 51-year-old man, obtained during microantrotomy. IHC for a-SMA. Magnification 200X. Cells with positive expression of a-SMA (indicated by arrows).
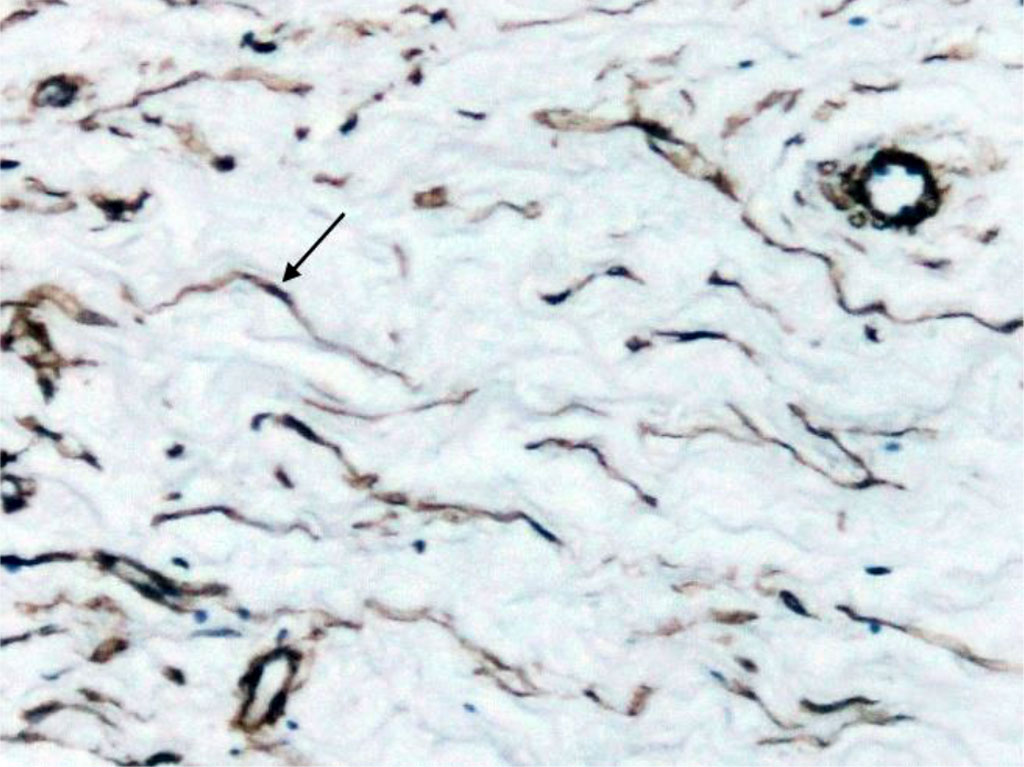
Fig. 4. Section of Schneiderian membrane from the anterior wall of the maxillary sinus of a 56-year-old man, obtained during microantrotomy. IHC for a-SMA. Magnification 400X. Cells with positive expression of a-SMA (indicated by arrows).
Analysis of the publications has shown that it is common to distinguish two populations of myofibroblasts depending on their localization: interstitial and subepithelial [15]. These two populations of cells have the same precursor and exist in the form of a syncytium. Therefore, we do not exclude such a connection within the Schneiderian membrane, which, in addition to the form-generating function, participates in the regulation of the cyclicity of changes in resistance to air flow, helping smooth myocytes in the venous sinuses. At the same time, the elasticity inherent in myofibroblasts only confirms our assumption. Besides, we should not ignore the guiding role of these cells in the migration of the epithelial layer during the formation of the maxillary sinus in the course of postnatal organogenesis. Myofibroblast-like cells engaged not only in the directed differentiation of cells, but in the development of fibrosis as well, which is often encountered in the practice of otolaryngologists.
It is important to note that all cells (endothelial cells, smooth myocytes and myofibroblast-like cells) that are presented in our study are capable of making the transition to the osteogenic line of differentiation [16]. Thus, the obtained data allow us to state that the Schneiderian membrane covering the maxilla from the side of the maxillary sinus contains cells that can potentially, under certain conditions, become a source of bone tissue formation. At the same time, the osteoprogenitor function of the periosteal coverage is reduced to a minimum, because the inner layer in the periosteal coverage is not expressed.
Microscopic examination of the Schneiderian membrane obtained during microantrotomy for removal of foreign bodies revealed that the mucosa of the sinus wall and the periosteum are in close structural and functional connection with each other due to the fibers of the extracellular matrix of the lamina propria of the mucosa, which are woven into the periosteal coverage. The periosteal coverage consists of a fibrous layer that looks like a single bundle of collagen fibers with single small cells that have positive expression to smooth muscle actin, but are not involved in the division processes. In addition to epithelial cells, connective tissue cells of the lamina propria and vascular endothelium are involved in the proliferation processes. These cells are capable of being a source of osteoprogenitor cells only under certain conditions. To their number we can add cells with positive a-SMA expression. Considering the functional profile of such cells, we believe that in the maxillary sinus they regulate cyclic changes in the degree of swelling of the mucosa, can play a directing role in the migration of the epithelial layer during pneumatization of the maxillary sinus and be the cause of fibrosis development.