- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 13; 1: e1. DOI 10.35630/2024/14/1.108
Parkinson's disease (PD) is a chronic, neurodegenerative disease belonging to the group of alpha-synucleinopathies. It is associated with the loss of dopaminergic neurons and is characterized by the presence of Lewy bodies. The diagnosis of PD is mainly based on the clinical picture. The basis of therapy are dopaminergic drugs, and the doses of drugs, as well as the frequency of their administration, should be increased along with the progression of the disease, which, however, is associated with an increase in side effects. We are still looking for new solutions that will make the therapy optimal and tailored to each patient. Currently, methods such as deep brain stimulation are becoming more and more available. New technologies such as PD-watch, hydrogel, Parkinson's gloves, STAT-ON TM or gene therapy bring hope to patients struggling with this disease on a daily basis.
The aim of our work was to provide an overview of modern treatment methods and to provide the reader with basic information about Parkinson's disease. The information we used in this work comes from a critical review of the Pubmed literature, as well as specialized medical literature.
Methods:We analyzed scientific publications containing information on Parkinson's disease, current treatment data. We also searched for modern diagnostic methods and information on Parkinson's disease treatment options. We have collected the data and summarized it in this article.
Conclusion: Currently available therapies provide good control of motor symptoms, but do not modify the development of Parkinson's disease. However, the development of technology and medicine allows us to think that in the future the fate of patients with this disease will change, and new solutions will bring positive results.
Keywords: Parkinson’s disease, deep brain stimulation, PD-watch, hydrogel, Parkinson’s gloves, levodopa, gene therapy.
Parkinson's disease (PD) is a chronic, slowly progressive, neurodegenerative disease belonging to the group of alpha-synucleinopathy. [30]
Worldwide, over 10 million people are affected, while in Europe the prevalence and incidence are estimated at approximately 108-257/100,000 and 11-19/100,000 per year, respectively.[3],[7]
PD affects 1-2% of the population of people over 65 years of age and may increase up to 4% among people over 85 years of age, which confirms the fact that the main risk factor for the development of the disease is old age, because cells show a greater degree of dysfunction during aging. [20].The incidence of the disease is slightly higher in men than in women.[29]. Familial forms of Parkinson's disease account for 5-15% of all cases.[3]
PD is associated with the loss of dopaminergic neurons, initially the dorsal nucleus of the vagus nerve and the olfactory bulb, then the locus coeruleus, and finally involving the substantia nigra.[20],[30]
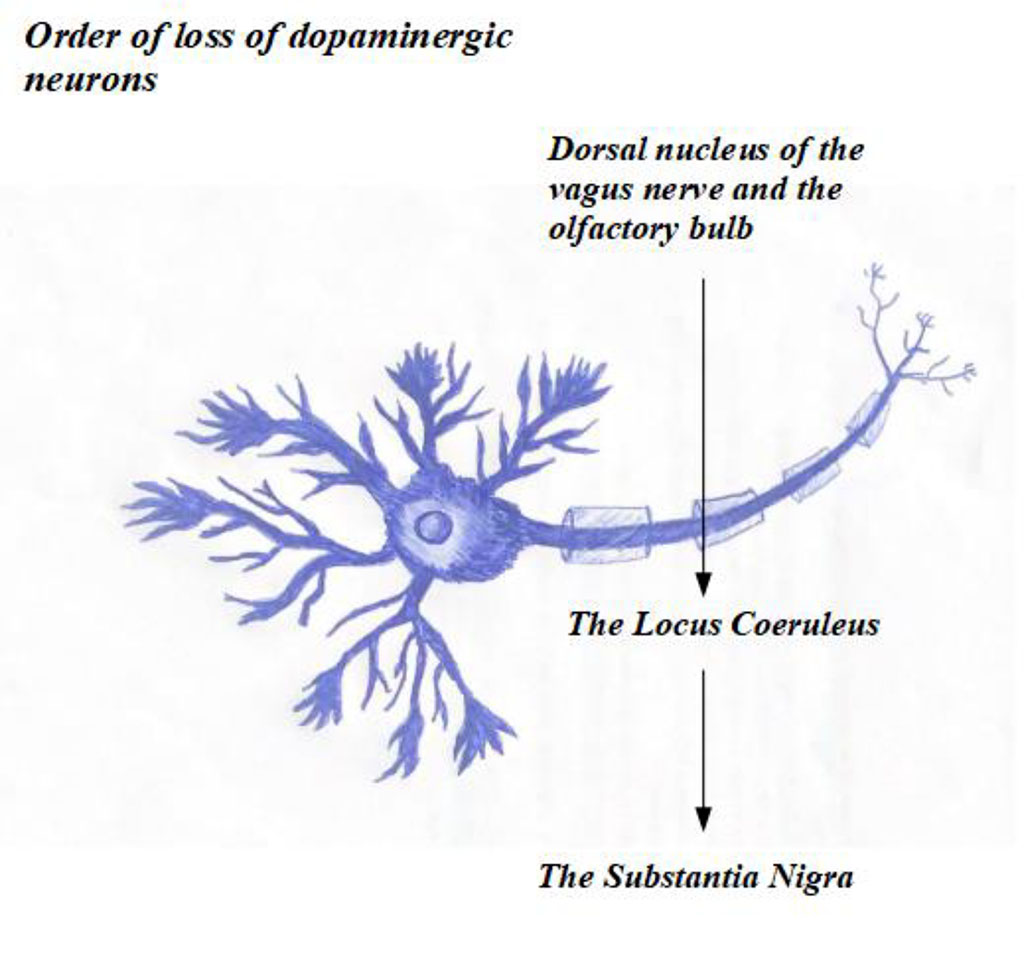
The extensive process of creating new neuronal processes of nigrostriatal dopaminergic neurons increases their energy demand and affects proteostasis and lysosomal functions. The loss of dopaminergic neurons in familial forms of the disease is associated with mutations in the SNCA, Parkin/PARK2, UCHL1, PINK1, DJ-1/PARK7, and LRRK2 genes.[2]
The disease is characterized by the presence of Lewy bodies, which are both the basic histopathological unit, as well as neuropathological changes occurring in the affected cells. They include α-synuclein and cell membrane components.[2],[30]
Various mechanisms at the cellular and molecular level also underlie the disease, including oxidative stress, mitochondrial dysfunction, proteostasis and neuroinflammation.[2]
Environmental risk factors for the development of the disease include alcohol, tobacco and exposure to vitamin D. Research indicates that, however, they have a smaller role in the pathogenesis.[29]
The first motor symptoms of Parkinson's disease occur as a result of the advanced degenerative process of the substantia nigra. In the next stage, the disease also affects cortical areas. The pathological process involves the central nervous system. Lewy bodies are also observed in the peripheral nervous system - in the sympathetic ganglia and the intramural gastrointestinal plexus. Their presence in these areas is responsible for vegetative symptoms in people with PD.[30]
Currently available therapies provide good control of motor symptoms but do not modify the development of the disease.[3]
Due to the increasing number of patients in Poland, work is underway to introduce comprehensive, coordinated care for patients with Parkinson's disease. The team would include neurologists, family physicians, as well as radiologists, physiotherapists, psychologists, speech therapists, dieticians and gastroenterologists. There would also be more emphasis on educating patients and their families. These activities will enable an early diagnosis and the patient to receive optimal therapy to ensure adequate quality of life.[27]
We have analyzed in detail the specialist literature and scientific articles on Pubmed regarding both Parkinson's disease and modern methods of its treatment. We have collected all the most important information about the disease as well as the possibilities of its diagnosis and treatment. Then we focused on modern, less popular methods of therapy and described them in detail.
Parkinson's disease is diagnosed mainly on the basis of its clinical picture. The diagnosis is based on criteria that include axial symptoms, additional symptoms and exclusion criteria[25],[10]. The value of these criteria is high, but the patient's examination must be performed by experienced and qualified personnel. In a study conducted on the population of general practitioners in Wales, it was found that the frequency of correct diagnosis was approximately 53%, which shows how important it is to properly train the diagnosing doctor. Family doctors who made an incorrect diagnosis in this study most often diagnosed essential tremor, Alzheimer's disease or vascular parkinsonism (parkinsonism of the lower half of the body without symptoms in the upper limbs) as Parkinson's disease [25], [24].
They may indicate, among others: multiple sclerosis, cerebellar stroke
| Symptoms of Parkinson's disease | ||
| Prodromal Symptoms | Axial Symptoms | Additional symptoms |
| Depression | Bradykinesia | Orthostatic Hypotension |
| Constipation | Muscle Stiffness | Psychomotor Retardation |
| Paresthesia of Limbs | Resting Tremor | Unstable Posture |
Neuroimaging:
It is mainly used to exclude other causes and is not used to confirm the diagnosis of Parkinson's disease
According to some authors, the combination of DaTSCAN with 18F-dopa PET and automated image analysis makes it possible to classify patients with Parkinson's disease with a specificity of 94-97%. [19]
The basis of Parkinson's disease therapy are dopaminergic drugs.[5] Currently, the most effective drug is the precursor of dopamine - levodopa, taken together with carbidopa, which is a peripheral inhibitor of levodopa decarboxylase.[22] An alternative therapeutic option to levodopa are dopamine agonists, monoamine oxidase B (MAO-B) inhibitors and, in some cases, anticholinergic drugs. Levodopa, which is a direct precursor of dopamine, unlike it, crosses the blood-brain barrier. It is used in combination with carbidopa, which prevents the peripheral conversion of levodopa to dopamine. The benefits of using levodopa include improvement in motor functions, extension of the period of ability to work and independent functioning.[26] Contrary to popular opinion about the benefits of delaying levodopa therapy due to its alleged toxicity, it is recommended to start treatment immediately.
It has been shown that people who start therapy quickly have a better quality of life and have fewer motor symptoms compared to people who decided to postpone it for 9 months from the onset of the disease. [5]. However, levodopa therapy in the early stages of the disease should involve the use of the lowest possible dose, because the need for the drug increases with the duration of treatment. At the beginning of therapy, it is recommended to combine 25 mg of carbidopa with 100 mg of levodopa, at least 3 times a day at intervals of at least 4-5 hours. Most patients do not need to take medications at night. Drug doses and their frequency should be increased as the disease progresses. However, as their dosage increases, the frequency of side effects of therapy, such as dizziness or gastrointestinal disorders, increases. In order to avoid them, despite the reduction in therapeutic effectiveness, it is possible to take levodopa with meals. Protein products, being competitive inhibitors of levodopa, reduce its effectiveness and the patient should be informed about this. If nausea occurs during levodopa treatment, it is recommended to use carbidopa, ondansetron, domperidone or trimethobenzamide, because antiemetic drugs such as metoclopramide, promethazine or prochlorperazine, through their antidopaminergic effect, may worsen the symptoms of Parkinson's disease. As the doses of levodopa increase, the incidence of complications of its use increases, such as motor symptoms, dyskinesia, orthostatic hypotension, hallucinations and delusions. To reduce fluctuations in drug concentration in the body, prolonged-release levodopa drugs have been introduced on the market.[22]
Dopamine agonists (DA) are a group of drugs also used in the treatment of Parkinson's disease. These drugs act directly on dopamine receptors in the brain, imitating the action of dopamine and thus effectively controlling the symptoms of the disease.[15],[22] These are preparations that penetrate well into the central nervous system and the intestine. This group of drugs is used as monotherapy at an early stage of the disease, especially in patients whose symptoms appeared before the age of 65. Thanks to this, it is possible in some cases to delay the implementation of levodopa treatment and avoid early motor complications in the patient, such as dyskinesia, fluctuations, dystonia. [15],[26] A big advantage of using dopamine agonists, especially those with long-term action, is the possibility of infrequent dosing of the drug. In oral form, drugs are usually used at least three times a day, and in the form of patches once a day. It is also possible to administer the drug apomorphine as a continuous intravenous infusion or as an injection. Dopamine agonists, unfortunately, show weaker therapeutic effects compared to levodopa treatment and are associated with numerous side effects.[15],[22] These include, among others, drowsiness, peripheral edema, psychotic symptoms, orthostatic hypotension, and valvular defects. A dangerous side effect is also impaired impulse control, which results in, for example, excessive eating, pathological gambling or shopaholism.[15],[26] In order to minimize the risk of the above complications, pharmacotherapy begins with small doses of the drug and then gradually increases its amount. [15]
Monoamine oxidase type B (MAO-B) inhibitors are drugs that interfere with the metabolism of dopamine by inhibiting the action of enzymes that inactivate dopamine. Thanks to this, its amount in the brain increases. MAO-B includes, among others: selegiline, rasagiline and safinamide. These drugs can be used both as monotherapy and in combination with levodopa. However, it is important to remember to reduce the amount of foods containing tyramine during MAO-B pharmacotherapy due to possible side effects. The most common symptoms include nausea, dizziness, headaches, and insomnia. When monoamine oxidase type B inhibitors are used with tricyclic antidepressants and SSRIs, it may cause convulsions, disturbances of consciousness and, in some cases, hypertensive crisis. Side effects may also occur when MAO-B is combined with painkillers such as tramadol or pethidine.[22]
The key to effectively fighting Parkinson's disease is to thoroughly understand its mechanism and holistic therapeutic measures. The combination of medical knowledge with the dynamically developing biotechnology and genetic engineering offers many new perspectives in the early detection of PD, stopping the progression of the disease, minimizing side effects, and even in the long term, complete recovery.
Deep brain stimulation (DBS) has become one of the recognized therapeutic options in the treatment of patients with Parkinson's disease (PD). Currently, it is considered in patients with progressive motor symptoms resistant to previously used therapy or with side effects of levodopa that make functioning difficult. The average duration of disease and pharmacological therapy before starting deep brain stimulation in Parkinson's disease is currently approximately 13 years. Research is still ongoing on the period of the disease that would be the optimal moment for electrode implantation and would give patients the opportunity to maintain an appropriate quality of life.[11],[18]
To obtain maximum benefit from deep brain stimulation in PD, careful patient selection is necessary to assess who will respond to treatment and achieve optimal therapeutic effect. For this purpose, a multidisciplinary team is established including a neurologist, a neurosurgeon, specialized nurses, and sometimes a neuropsychologist. Inclusion criteria for DBS are: symptomatic, idiopathic Parkinson's disease, significant improvement in the clinical condition compared to previously used dopaminergic drugs (<30% of patients), treatment-resistant motor fluctuations or tremor, only symptoms from the group of minor criteria in the ON state. Relative criteria for exclusion from therapy include: biological age over 75 years, severe/malignant comorbid disease with significantly reduced life expectancy, chronic immunosuppression, and marked brain atrophy. Atypical parkinsonian syndromes, such as multiple system atrophy or progressive supranuclear palsy, show only slight and transient improvement and should not be treated with deep brain stimulation.[11],[18]
Current evidence suggests that, from a neurophysiological point of view, motor symptoms in PD result from dysfunction of one or more basal ganglia thalamocortical connections.
In DBS therapy, standard stimulation of the subthalamic nucleus (STN) and globus pallidus interna (GPi) is performed using electric current generated by an implantable pulse generator (IPG). The implanted electrodes produce a constant, pre-programmed output stimulation. Experimental work in primates and humans has shown that DBS (STN) interacts with diseased neural networks in multiple ways, stimulating some pathways while inhibiting others.[11],[18]
The operation is performed in a conscious patient using a stereotaxic frame (e.g. Leksell frame) mounted around the patient's head. Computed tomography (CT) and magnetic resonance imaging (MRI) are used for intraoperative imaging. The CT/MRI scanner computer uses software to spatially integrate the stereotaxic frame with the CT/MRI images and the scanner gantry to determine brain coordinates as well as calculate potential probe paths , which helps achieve accuracy of 1 mm.[11],[18]
Stimulation of the STN and GPi allows for the reduction of tremor, bradykinesia and stiffness. Work is underway to stimulate the peduncular nucleus (PPN), which would reduce patient symptoms such as postural instability and gait difficulties, which could not be achieved with STN stimulation.[11],[18]
DBS therapy is expensive - the costs of equipment, use of operating facilities, staff and hospital bed occupancy must be taken into account, which is not required by the constant use of pharmacotherapy. Nevertheless, European studies showed that the average cumulative 5-year cost per patient was significantly lower for DBS (EUR 88,014) compared to continuous subcutaneous infusion of apomorphine (EUR 141,393) or continuous duodenal infusion of levodopa or carbidopa (EUR 233,986 ) (P = <0.0001).[18]
When considering the introduction of DBS, it should be taken into account that, like other therapies used to treat Parkinson's disease, it is not possible to stop the progressive neurodegenerative process that underlies PD.[11],[18]
The Italian company Biomedical Lab, with EU support, has designed an innovative device as part of the PD-Watch project that combines a motion-detecting sensor and software that allows you to distinguish normal body movements from those occurring as one of the main symptoms of Parkinson's disease. Hand tremors in PD occur at frequencies between 4 and 6 Hz and are additionally accompanied by impaired supination-pronation. The modern technology contained in PD-Watch allows for early detection of the first symptoms of Parkinson's disease and 24-hour monitoring of already diagnosed patients, which allows for the assessment of therapeutic effects. Thanks to this, the neurologist is able to optimize the treatment after reading the information from the device in order to reduce motor symptoms in patients with PD as much as possible. The PD-Watch device should help in routine clinical practice and alleviate the suffering of the growing number of Parkinson's disease patients through a tailor-made therapeutic plan.[1]
Scientists from the Australian National University, in collaboration with the Florey Institute of Neuroscience and Mental Health, have developed a hydrogel made of amino acids that could be used as a one-off intervention in the treatment of Parkinson's disease. One of the main problems in PD treatment concerns the delivery of drugs to the CNS, mainly due to the blood-brain barrier. The hydrogel may be a breakthrough solution as a drug carrier, offering one of the most attractive therapies for Parkinson's disease.
After injection, the hydrogel turns into a liquid, making it easier to introduce it through the capillary into the brain. The gel then returns to its original consistency and fills the empty spaces and facilitates the transport of stem cells to the damaged parts of the brain. Stem cells have been subjected to numerous studies for years with the hope of using them in the treatment of neurodegenerative diseases. Currently, the hydrogel is still in the research phase. [6],[13]
Doctors from King Chulalongkorn Memorial Hospital developed the so-called "Parkinson's gloves", which after being patented were named "Parkinson's gloves reducing tremors". Their goal is to automatically reduce hand tremors that PD patients struggle with every day, which will significantly improve their functioning and comfort. The mechanism of action of this invention consists in electrical stimulation of the hand muscles by combining two mechanisms: detecting and measuring muscle tremors using an accelerometer and gyroscope with the simultaneous release of electric current by the muscle stimulator at an appropriate intensity that is safe for the body. As a result, one of the main symptoms of Parkinson's disease is significantly reduced.[12]
The STAT-ON™ device monitors the symptoms of Parkinson's disease objectively, providing a complete map of their occurrence and severity. It measures motor disturbances and events in PD patients, but does not measure tremor. It records all involuntary movements, gait pattern, falls, energy expenditure and posture. Advanced algorithms analyze the information and identify specific symptoms of Parkinson's disease such as bradykinesia, dyskinesia, freezing while walking or motor fluctuations. Data from the sensors is presented in the form of a report used by the neurologist to make treatment decisions based on information from the device. For the patient, the use of STAT-ON ™ is very simple: it is worn around the waist, and the data is automatically saved on the smartphone.[23]
Many modern solutions are still in the research phase and some of them may not be widely available yet.
With new technologies, their growing power and versatility, and intelligent algorithms that the world of medicine increasingly relies on, the future of diagnostics, treatment and improving the quality of life of patients with neurodegenerative diseases holds much hope.
Technology progress and newer concepts have made it possible to implement new, non-schematic variants of adenoviral vectors (AAV). Using adenoviruses as vectors is very useful because of their unique action. Many PD clinical trials have shown that AAV is a suitable and, most importantly, safe vector for gene therapy. Of course, other viruses can also serve as vectors, but AAV has several important features that outclass other competitors in the field of non-dividing cell therapy. An adenovirus is a dependent virus, i.e. it requires another adenovirus to replicate, so it gives it "information" regarding the replication processes. This mechanism can be bypassed by transient transfection of plasmids. This situation allows us to focus on only two genes: the Rep replication gene and the Cap gene responsible for capsid synthesis. These genes can be easily extracted from the adenovirus genome and transferred to a plasmid. Due to this transient transfection, we obtain an empty AAV vector where only the inverted terminal repeats (ITRs) remain present. After isolating the Rep and Cap genes, we need to fill the remaining gap, for this purpose we use a therapeutic gene (promoter and regulatory sequences) that will stabilize the mRNA. Side effects are also possible due to genome remnants and empty capsid, as they are low immunogenic. This should always be kept in mind because the human body has antibodies against the genome and capsid.
Depending on the type of administration route, we must select appropriate variants of capsid structure in order for AAV vectors to reach the basal ganglia.
There are four main routes of delivery during AAV therapy in Parkinson's disease:
[4]
Gene therapy is becoming more and more advanced every year. Let's hope that the next years will bring new innovations that will result in greater effectiveness and reduced incidence of side effects of the therapy used in patients, or even provide a complete cure for Parkinson's disease.
Parkinson's disease is a chronic, slowly progressive neurodegenerative disease. A number of genetically and metabolic processes are responsible for its occurrence, and diagnosis is mainly based on the clinical picture. Currently available therapies provide good control of motor symptoms but do not modify the development of the disease. However, with the development of medicine, we observe dynamic changes and many new studies on this issue, which brings hope for patients with Parkinson's disease.
The aim of the study was to collect information and summarize treatment options for patients with this disease.