- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 13; 1: e1. DOI 10.35630/2024/14/1.105
The purpose of this study was to evaluate the results of differentiated use of enteral antihypoxic therapy (EAT), endoportal therapy (EPT), as well as phage therapy (PT) in patients with diffuse purulent peritonitis complicated by acute intestinal failure (AIF).
Materials and Methods: 144 patients with diffuse peritonitis and AIF were treated. The patients were divided into two equal comparable groups: main (n=72) and comparison (n=72). In addition, each group was divided into three subgroups (24 patients each) depending on the degree of AIF. The groups were distributed using adaptive randomization. EAT was carried out by introducing oxygenated water through a nasogastrointestinal (NGI) tube. EPT was carried out by introducing an antibiotic, a hepatoprotector and an antihypoxant through a catheter into the portal system. PT was carried out by introducing a polyvalent bacteriophage through a NGI tube. The following were dynamically studied: EMFC, APACHE II, abdominal cavity index (ACI), acid-base status and blood gas composition, integral intestinal pressure (IIP), intra-abdominal pressure (IAP). Comparison of quantitative indicators in the study groups was carried out using the Wilcoxon-Mann-Whitney U test, as well as its multivariate generalization - the Kruskal-Wallis test, for checking the equality of medians of several samples.
Results: The dynamic study of IIP and IAP in the study groups on the day 3 of treatment, showed significantly lower values in the main group (p≤0.05). There was a more rapid decrease in the manifestations of hypoxia in the main group, which was confirmed by an increase in the oxygen partial pressure in the blood. The average number of bed days in the intensive care unit was 4.2±0.3 days in the comparison group, and 3.3±0.2 days in the study group. The overall average number of bed-days was 15.8 in the study group, and 18.5 in the comparison group. Mortality was 16.3% in the comparison group, and 11.3% in the study group (a decrease of 30.7%).
Thus, when prescribing NGI, EAT, EPT and PT, one should be guided by the severity of organ dysfunction and the AIF stage. With values of ACI <13, APACHE II <10 and EMFC <5, NGI and EAT are indicated. With ACI 14-22, APACHE II 10-15, EMFC 5-25 - NGI and EAT are supplemented with PT. In the most severe cases, with ACI ≥23, APACHE II >16 and EMFC >25 points, EPT must be added to the above treatment methods.
Conclusion: The proposed scheme for the differentiated use of EPT, EAT and PT in patients with diffuse peritonitis complicated by AIF allows to relatively quickly stop inflammation and prevent the development of multiple organ failure and sepsis.
Keywords: peritonitis, acute intestinal failure, endoportal infusion, antihypoxic therapy, oxygenated water, phage therapy.
Secondary peritonitis and intra-abdominal sepsis are a global issue in public healthcare. The life-threatening systemic damage resulting from intra-abdominal sepsis has long been known, but remains insufficiently studied [1-5]. Direct surgical treatment for diffuse peritonitis is well developed and understood, however, there are not enough systemic treatment methods to control the subsequent systemic inflammatory reaction [6-8]. Recent advances in intensive care have led to improved outcomes in the treatment of secondary peritonitis. It should be noted that understanding changes in the human microbiome in secondary peritonitis is an emerging field and may lead to new potential therapeutic targets [9-13].
Acute intestinal failure (AIF) syndrome with peritonitis is a particularly difficult problem because the focus of the disease occurs within a limited cavity, where inflammation causes abnormally increased intra-abdominal pressure. The intestinal barrier is one of the most important components maintaining homeostasis in the gastrointestinal tract. Loss of its integrity due to changes in bacterial composition, decreased expression of tight junction proteins and increased concentrations of pro-inflammatory cytokines can lead to intestinal hyperpermeability with the subsequent development of many complications [14-18]. Increased permeability of the intestinal barrier may be the first step in the development of various complications, given that undigested food particles, bacterial toxins and microbes can pass through the “leaky” intestinal wall primarily into the portal circulation, triggering the immune system and causing persistent inflammation. Therefore, therapeutic effects on the routes of infection, for example, the portal system, are an important task [19-21].
Given that, in AIF, enterocytes function under hypoxic conditions, there is a need for therapeutic antihypoxic effects on the intestinal wall [22-24]. Recent clinical reports have described the therapeutic effects of oxygenated water on various diseases such as obesity, diabetes, liver diseases, cancer and surgical infection. In addition, there are earlier studies on the effectiveness of oxygenated water in anaerobic infection. Probably one of the effects is the creation of unfavorable conditions for the growth and reproduction of obligate anaerobes [25, 26]. The data obtained require further study.
The widespread prevalence of resistance to antibacterial drugs and the need to find safer alternative treatment methods have prompted research on the use of bacteriophages. In the search for alternative strategies for the prevention and control of bacterial infection, attention is increasingly focused on phage therapy (PT) [27-30]. Proponents of this therapy highlight several main advantages of PT over antibiotic therapy: specificity, self-propagation, the ability to destroy microbial biofilms, and the absence of organ and systemic toxicity. The clinical effectiveness of the use of phages has been confirmed in the treatment of patients with acute destructive pancreatitis and pancreatic necrosis, as well as infectious complications in abdominal surgery. To improve the antimicrobial effect, it is necessary to constantly monitor the sensitivity of microflora to drugs of bacteriophages and constantly update drugs for PT [31-34].
It should be noted that clinicians have great problems recognizing AFI, since some symptoms coincide with other common gastrointestinal diseases and complications, and, in addition, the symptoms of the underlying disease may mask the symptoms of enteral insufficiency. It should be noted that there is no gold standard procedure to clearly characterize the barrier function of the intestinal wall. Currently available barrier function tests measure very different endpoints and therefore their clinical significance and relevance are unclear and require clarification.
The purpose of the study was to evaluate the results of differentiated use of enteral antihypoxic therapy (EAT), endoportal therapy (EPT), as well as PT in patients with diffuse purulent peritonitis complicated by AIF.
144 patients were treated after surgery in the general surgery clinic for various abdominal pathologies complicated by general peritonitis and AIF. The average age of the patients was 44.7±13.3. There were 74 men and 70 women. The patients were divided into two equal comparable groups: main (n=72) and comparison (n=72). In addition, each group was divided into three subgroups (24 patients each) depending on the degree of acute intestinal failure. The groups were distributed using adaptive randomization.
EAT was carried out by introducing oxygenated water (OW) (sterile purified water saturated with oxygen in an amount of at least 250,000 ppm) through a nasogastrointestinal (NGI) tube. OW was administered intraoperatively, and then 200 ml 2 times a day, followed by clamping of the tube for 60 minutes for 3-5 days, followed by removal of the tube orally.
EPT was carried out by introducing drugs through a catheter: meglumine sodium succinate 10 ml/kg/day, ciprofloxacin 400 mg/day and ademetionine 400 mg/day for 3-5 days at a rate of 60 drops per minute. There were no complications after catheterization and endoportal infusions [35].
Enteral PT was carried out by introducing a polyvalent bacteriophage, which is sterile purified filtrates of phagolysates of bacteria Staphylococcus spp., Streptococcus spp., Proteus (P. vulgaris, P. mirabilis), Pseudomonas aeruginosa, Klebsiella pneumoniae, and enteropathogenic Escherichia coli (with Appelman activity at least 10-5) through a NGI tube - up to 1 ml. Bactriophage was injected into the small intestine 20 ml 3 times a day for 3-5 days.
All patients underwent nasogastrointestinal (NGI) drainage. The following were dynamically studied: enteral morpho-functional coefficient (EMFC), APACHE II, Savelyev's abdominal cavity index (ACI) (during primary surgery, relaparotomy and routine sanitation), acid-base state and gas composition of the blood, integral intestinal pressure (IIP), intra-abdominal pressure (IAP) and a number of general clinical criteria. Tab 1.
Table 1 Distribution of patients in the study groups by severity of condition and treatment methods
| AFI stage | Severity rating scales (points) | Study groups (n=144) | |||
| EMFC | ACI | APACHE II | |||
| Main group (n=72) | Comparison group (n=72) | ||||
| I | < 5 | < 13 | < 10 | 24** | 24* |
| II | 5-25 | 14-22 | 10-15 | 24*** | 24* |
| III | >25 | ≥23 | ≥16 | 24**** | 24* |
Treatment methods: * – NGI; ** – NGI+EAT; *** – NGI+EAT+PT; **** –NGI+EAT+EPT+PT
Statistical analysis was carried out using the “Statistica” v.6.0 program. Comparison of quantitative indicators in the study groups was carried out using the Wilcoxon-Mann-Whitney U test, as well as its multivariate generalization - the Kruskal-Wallis test, for checking the equality of medians of several samples.
During the dynamic study of IID in the study groups, on the day 2 of treatment, significantly lower values were noted in the main group (82±2.3 mm H2O). At the same time, in the comparison group this indicator was 110±2.5 mm H2O (p≤0.05). On the next day, no statistically significant differences in IID were noted between the groups (p≥0.05). Fig. 1.
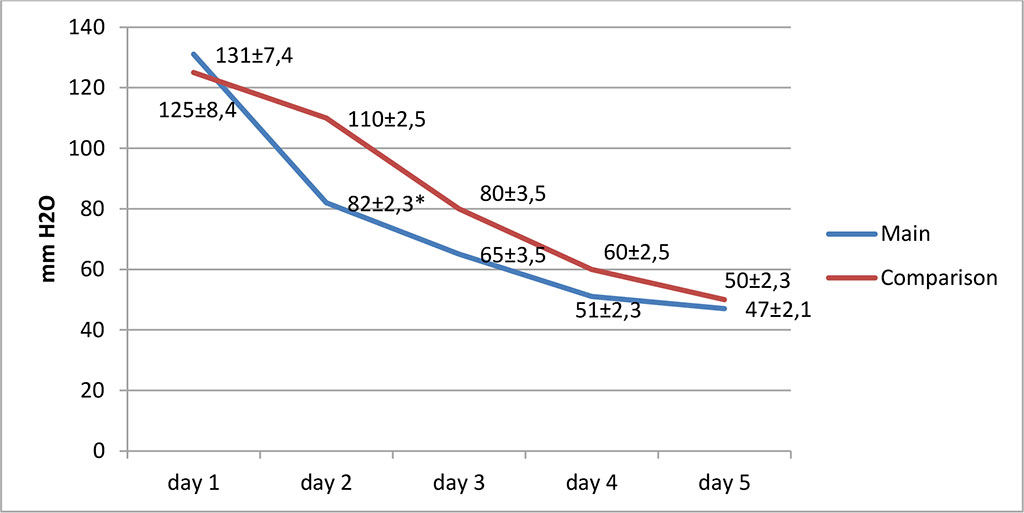
Figure 1. Change in IID in the study groups (* – values at p≤0.05)
The IAP values at the start of treatment were significantly higher than normal values in both study groups and corresponded to stage III of intra-abdominal hypertension. Fig. 2.
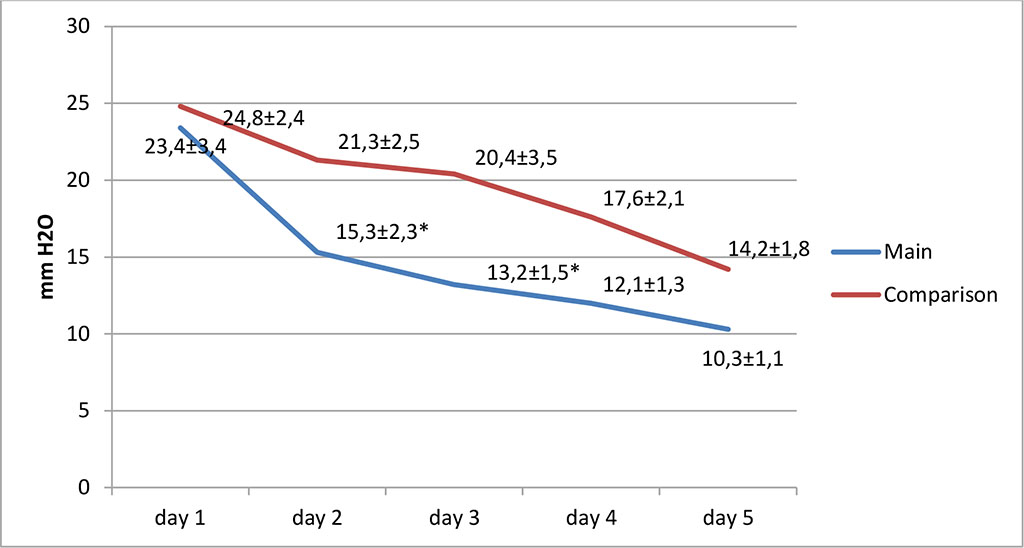
Figure 2. Change in IAP in the study groups (* – values at p≤0.05)
Further, on the days 2 and 3 of treatment, in the main group, we noted a significant decrease in the level of IAP to stage I of intra-abdominal hypertension (p≤0.05). At the same time, in the comparison group, the level of IAP corresponded to stages II-III. On the day 5, in both groups, IAP levels did not reach normal values (5-7 mmHg).
A dynamic study of the gas composition and acid-base balance of the blood revealed that all patients in both groups with diffuse peritonitis and AIF had acidosis, hypercapnia and hypoxia.
The pH value in both groups varied from 7.1 to 7.32 and did not return to normal values during intraoperative monitoring. The level of partial pressure of carbon dioxide (pCO2) at the beginning of the operation was above the normal value in the main group and the comparison group and amounted to 59.5 ± 3.5 mmHg and 58.2±4.9 mmHg respectively. Further, during the surgical intervention, the partial pressure of carbon monoxide was comparatively lower in the main group, however, no statistically significant difference was found compared to the comparison group (p≥0.05).
The values of oxygen partial pressure in the blood (pO2) before the start of EAT in both groups were below normal values and were not statistically different - they amounted to 34±1.1 mmHg and 36±0.9 mmHg in the main and comparison groups, respectively. Further, after 30, 60 and 120 minutes, in the main group, pO2 values were significantly higher than in the comparison group and amounted to 63±1.2 mmHg, 68±1.1 mmHg and 81±1.5 mmHg respectively.
Initial values of hemoglobin saturation with oxygen (SO2), as well as the indicator - O2 std., were low in both study groups. Further, in a dynamic study during surgery, these values were significantly higher in the main group (p≤0.05).
Thus, there was a more rapid decrease in the manifestations of hypoxia in the main group, which was confirmed by an increase in the partial pressure of oxygen in the blood.
When analyzing the severity of the condition of patients with AIF stage I in the main group, a statistically significant normalization of indicators of ACI and APACHE II was seen on days 5-6 of treatment (p≤0.05). The EMFC indicators in the main group and the comparison group showed no statistical difference (p≥0.05).
In patients with AIF stage II in the main group, there was a statistically significant decrease in ACI and EMFC indicators on days 3-4 relative to the comparison group (p≤0.05). On days 5-6, all three scales (ACI, APACHE II and EMFC) showed a statistically significant decrease in sum of the points in the main group (p≤0.05)
In the group with AIF stage III, there was a statistically significant decrease by all three studied scales on days 3-4 of treatment (p≤0.05). And then on days 5-6, this trend continued, i.e. there was a significant decrease in the average values of ACI, APACHE II and EMFC indicators (p≤0.05).
A dynamical study of the severity of the patients’ condition by the APACHE II scale, the condition of the abdominal organs by the ACI (V.S. Savelyev’s) and EMFC, revealed that in the comparison group, when using only NGI drainage, the indicators did not differ significantly. At the beginning of treatment and further on days 2-3 and 5-6, a decrease in indicators was noted, however, they did not return to normal values.
In the main group, in patients with AIF stage II-III, already by the day 3, a statistically significant decrease in the studied markers was noted, which indicated a more rapid relief of the syndrome of multiple organ dysfunction and AIF, as well as a subsidence of inflammatory changes in the abdominal organs. See tab. 3.
Table 3 Dynamics of severity of patients’ condition in the groups (М±m)
| Treatment methods | Scales | Duration of treatment/AIF stages | ||||||||
| AIF stage I (n=48) | AIF stage II (n=48) | AIF stage III (n=48) | ||||||||
| Day 0-1 | Day 3-4 | Day 5-6 | Day 0-1 | Day 3-4 | Day 5-6 | Day 0-1 | Day 3-4 | Day 5-6 | ||
| NGI (n=72) | ACI | 12,8±0,9 | 12,3±1,1 | 9,2±1,2 | 19,2±2,3 | 16,8±1,3 | 10,6±0,3 | 24,2±2,3 | 20,8±1,3 | 14,6±0,3 |
| APACHE II | 9,2±0,5 | 8,1±0,3 | 7,2±0,1 | 14,1±0,4 | 10,8±0,4 | 8,9±0,2 | 23,4±0,4 | 17,8±0,3 | 15,9±0,9 | |
| EMFC | 4,2±0,3 | 3,6±0,2 | 3,3±0,3 | 23,4±2,2 | 15,5±1,5 | 10,3±0,6 | 25,6±3,2 | 17,5±4,3 | 16,6±3,7 | |
| NGI+ EAT (n=24) | ACI | 12,6±0,6 | 10,6±0,5 | 5,3±0,6* | - | - | - | - | - | - |
| APACHE II | 8,6±0,6 | 9,5±0,2 | 4,4±0,4* | - | - | - | - | - | - | |
| EMFC | 4,4±0,2 | 3,8±0,4 | 3,1±0,3 | - | - | - | - | - | - | |
| NGI+ EAT +PT (n=24) | ACI | - | - | - | 20,0±1,3 | 11,8±0,4* | 5,5±1,5* | - | - | |
| APACHE II | - | - | - | 14,5±0,7 | 8,7±0,2 | 6,7±0,3* | - | - | - | |
| EMFC | - | - | - | 24,2±1,1 | 10,4±1,3* | 5,5±0,4* | - | - | - | |
| NGI+ EAT +PT+EPT (n=24) | ACI | - | - | - | - | - | 23,8±2,3 | 13,8±1,4* | 9,5±1,5* | |
| APACHE II | - | - | - | - | - | - | 24,5±0,3 | 10,7±0,5* | 9,7±0,3* | |
| EMFC | - | - | - | - | - | - | 26,5±3,3 | 11,8±2,2* | 9,3±1,3* | |
* - indicators with significance of changes compared to the NGI group at p ≤0.05
The average number of bed days in the intensive care unit was 4.2±0.3 days in the comparison group and 3.3±0.2 days in the study group. The overall average number of bed days was 15.8 in the study group and 18.5 in the comparison group. Mortality was 16.3% in the comparison group and 11.3% in the study group (a decrease of 30.7%).
Thus, when prescribing NGI, EAT, EPT and PT, one should be guided by the severity of organ dysfunction and the AFI stage. With values of ACI <13, APACHE II <10 and EMFC <5, NGI and EAT are indicated. With ACI 14-22, APACHE II 10-15, EMFC 5-25, NGI and EAT are supplemented with PT. In the most severe cases, with ACI ≥23, APACHE II >16 and EMFC >25 points, EPT must be added to the above treatment methods. Fig. 3, 4.
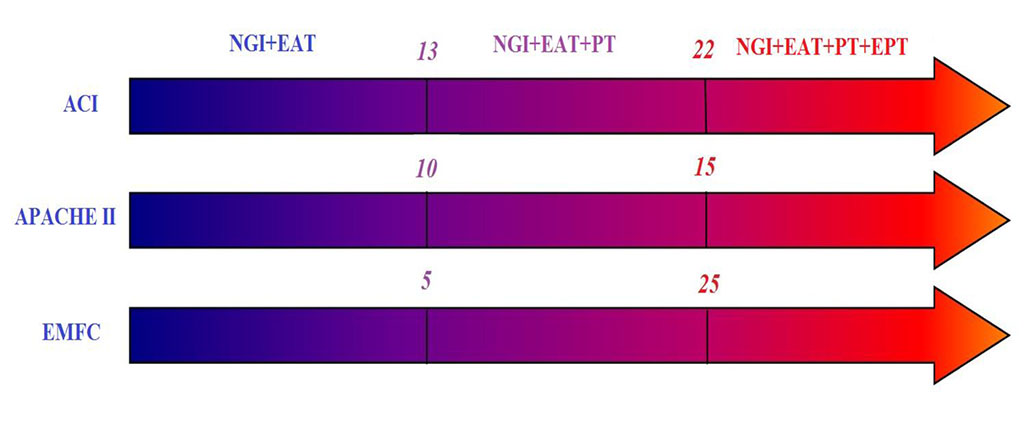
Figure 3. Scheme of differentiated use of treatment methods depending on the severity of multiple organ failure
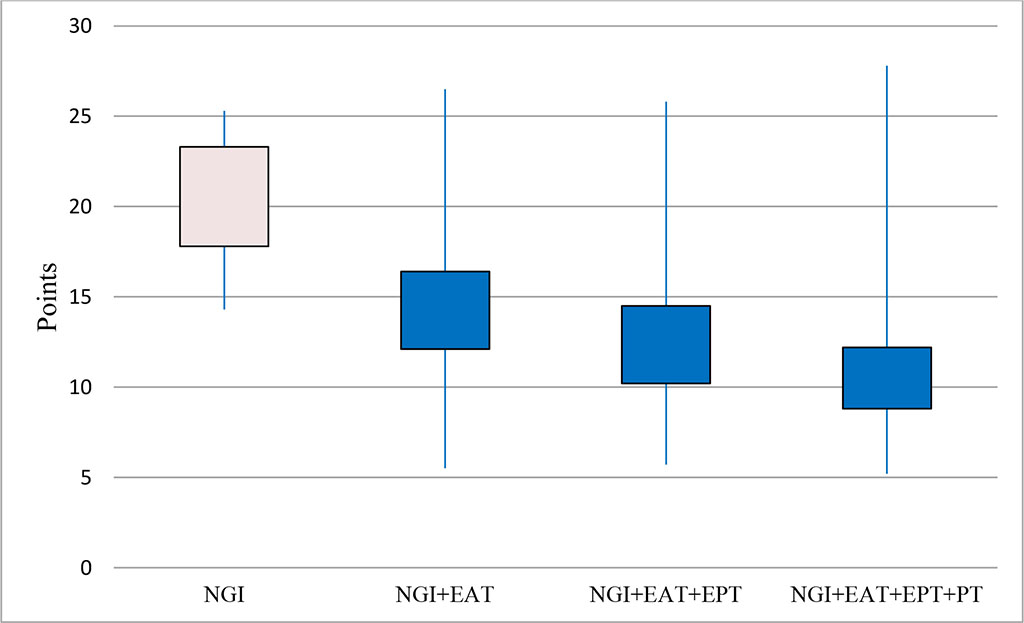
Figure 4. The effectiveness of the scheme of differentiated use of treatment methods (Kruskal-Wallis H test (3, N=144)=12.1 p=0.01)
Thus, the clinical results of the differentiated use of EPT, as well as EAT and PT in patients with diffuse peritonitis complicated by AIF, showed that the choice of methods and their combination depend on the severity of the patient’s condition.
The proposed scheme for the differentiated use of EPT, EAT and PT in patients with diffuse peritonitis complicated by AIF allows to relatively quickly stop inflammation and prevent the development of multiple organ failure and sepsis.