- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 13; 1: e1. DOI 10.35630/2024/14/1.104
The purpose of this study was to evaluate the effectiveness of EIT for intra-abdominal infections complicated by acute intestinal failure.
Materials and Methods: The authors modified the technique of catheterization of the right gastroepiploic vein to conduct prolonged endoportal infusion therapy. The modification allows for easy surgery and successful removal of the catheter upon completion of endoportal infusion therapy. This operation is possible using video laparoscopic access. An antihypoxic agent, an antibiotic, and a hepatoprotector were given as an endoportal bolus. The technique was carried out on 24 patients with various purulent-inflammatory diseases of the abdominal organs complicated by acute intestinal failure. The results were compared with a group of 24 patients treated with traditional methods.
Research Results: As a result of the study, it turned out that in the main group, indicators returned to normal according to the systems for assessing the severity of the condition (APACHE II and abdominal index) and signs of intestinal failure (EMFC) on the day 5 of treatment. At the same time, in the comparison group, these changes occurred only on the day 7 of treatment (p≤0.05). The values of enzymes reflecting the state of the intestinal wall, as well as indicators of cellular immunity were normalized by day 3 in the main group, and by day 7 in the comparison group (p≤0.05).
Conclusion: The proposed EIT technology can serve as a good supplement to the treatment of patients with intra-abdominal purulent-inflammatory diseases complicated by acute intestinal failure. It prevents bacterial translocation into the portal system and thus reduces the risk of generalized infection and multiple organ failure.
Keywords: intra-abdominal infection, acute intestinal failure, bacterial translocation, endoportal drug administration, catheterization, portal vein.
The main route of absorption of molecules coming from the gastrointestinal tract is venous outflow through the portal vein into the liver. This absorption route is often critical because it allows the liver to biotransform these molecules before they enter the peripheral circulation. In many cases, molecules delivered through the portal vein to the liver undergo extensive first-pass metabolism in the liver, also known as the first-pass effect, such that their biotransformed products are the predominant forms observed in the peripheral circulation. It is known that the portal blood metabolome can influence liver physiology in numerous diseases [1].
Thus, a route of drug administration that ensures that any given substance is delivered in a manner replicating natural delivery in vivo is of critical importance. Currently, common routes of administration include intravenous, intraperitoneal, subcutaneous, tube, or simple oral administration. Critically, none of these routes of administration guarantee that the administered drug will remain unchanged after intestinal digestion or intestinal microbial metabolism as it passes through the portal circulation to the liver to exert its therapeutic effects [2].
With oral, intramuscular and intravenous administration of drugs, they undergo biotransformation, their pharmacological properties change, the concentration of drugs before they enter the liver is significantly reduced, which reduces the therapeutic effect. In addition, they are administered in fractional doses, and their concentration is different at different times of the day. With endoportal infusion therapy (EIT), drugs can be administered long-term and continuously. This continuity of action is crucial in a number of diseases and their complications in abdominal pathologies, especially in peritonitis complicated by intestinal failure syndrome [3-5].
Bacterial translocation is considered a causative factor in postoperative sepsis. This fact suggests that intestinal microbiota coupled with altered intestinal barrier homeostasis may create a chain of events leading to sepsis, as commensal bacteria move into normally sterile tissues. Bacterial translocation was observed during both laparotomy and laparoscopic operations, without significant differences in incidence. Proper management and early interventions based on the fundamentals of sepsis treatment are necessary [6–8]. In the development of this concept in recent years, a new approach has emerged: pharmacological antihypoxants were included in the program of intensive care of intestinal failure syndrome. Antihypoxants actively stimulate organ metabolism with the introduction of medicinal compositions (antibiotic, antihypoxant, hepatoprotector) into the portal bloodstream. It is assumed that EIT will make it possible to relieve intestinal failure syndrome at an earlier time, prevent the translocation of pathogens into the portal circulation and eliminate multiple organ failure [9-11].
Endoportal catheterization of the recanalized umbilical vein was widely used in Russia in the late 60s-70s. Various drugs, antibiotics, hepatoprotectors, antihypoxants, etc. were administered endoportally. This technique was mainly used for liver diseases of a purulent-inflammatory and other nature [12].
The method of administering drugs through a recanalized vein, in addition to its advantages (relative non-invasiveness, extraperitoneal access), had a number of disadvantages. For example, as a number of authors note, it is not always possible to insert a catheter extraperitoneally. And this is explained not by a lack of experience in catheterizing the umbilical vein, but by its anatomical features. The umbilical vein is formed from the veins of the anterior abdominal wall. In addition to a large tributary, it usually has a number of small peri-umbilical veins that flow directly into it (the Vine-Dyen intercalary veins) [13-16]. The remaining canal, depending on the number and caliber of the branches flowing into it, in some cases will be passable for the probe, in others it will barely let a hair through. In such cases, extraperitoneal catheterization of the umbilical vein is impossible, and opening the peritoneum deprives this manipulation of its main advantage compared to catheterization of intra-abdominal tributaries of the portal vein, including the right gastroepiploic vein. In addition, as mentioned above, the characteristics of the confluence of the umbilical vein make it possible to saturate mainly the left lobe of the liver with drugs (≈ 25% of the liver volume) [17-19].
Based on this, we can conclude that medicinal substances injected into the right gastroepiploic vein will be evenly and completely distributed throughout the liver parenchyma.
The purpose of this study was to evaluate the effectiveness of EIT for intra-abdominal infections complicated by acute intestinal failure.
We performed catheterization of the right gastroepiploic vein using the generally accepted method, but with some of our own modifications. After laparotomy, the greater curvature of the stomach was exposed into the incision. At the level of the antrum, the right gastroepiploic vein was isolated. The latter was transected and the right end was mobilized in the direction of the portal vein by 3-5 cm, tying off all outlying branches. The opposite end was ligated or coagulated using the bipolar method. A Roeder's knot was formed through the right section of the vein using absorbable suture material. Then, a catheter (5F) was inserted through the mobilized section of the right gastroepiploic vein into the portal vein using the Seldinger technique. The catheter was fixed (not tightly, but hermetically) to the right gastroepiploic vein with a previously made Roeder’s knot. Fig. 1.
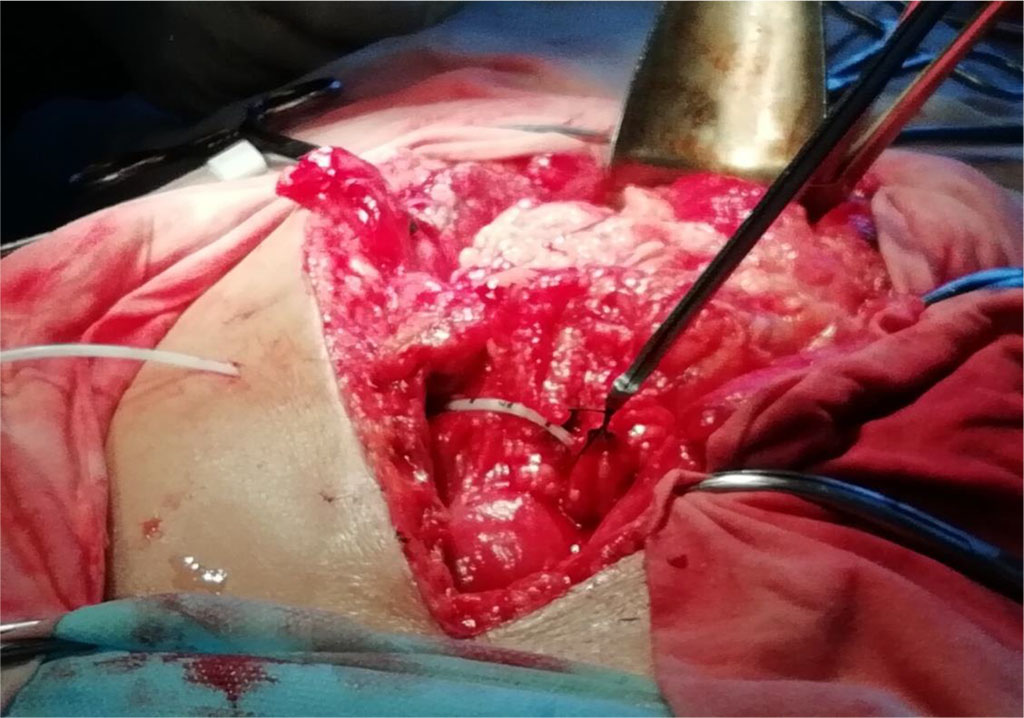
Figure 1 Fixation of the catheter in the lumen of the right gastroepiploic vein with the Roeder’s knot using a pusher (knot pusher)
After that, it was flushed with a heparin solution (2500 units per 80 ml of physiological solution), and an obturator line was inserted into the lumen. After catheterization, portography was performed using a Phillips Allure FD 20 angiographic unit. The intravascular position of the catheter in the portal system was assessed, followed by measurement of invasive pressure. Fig. 2.
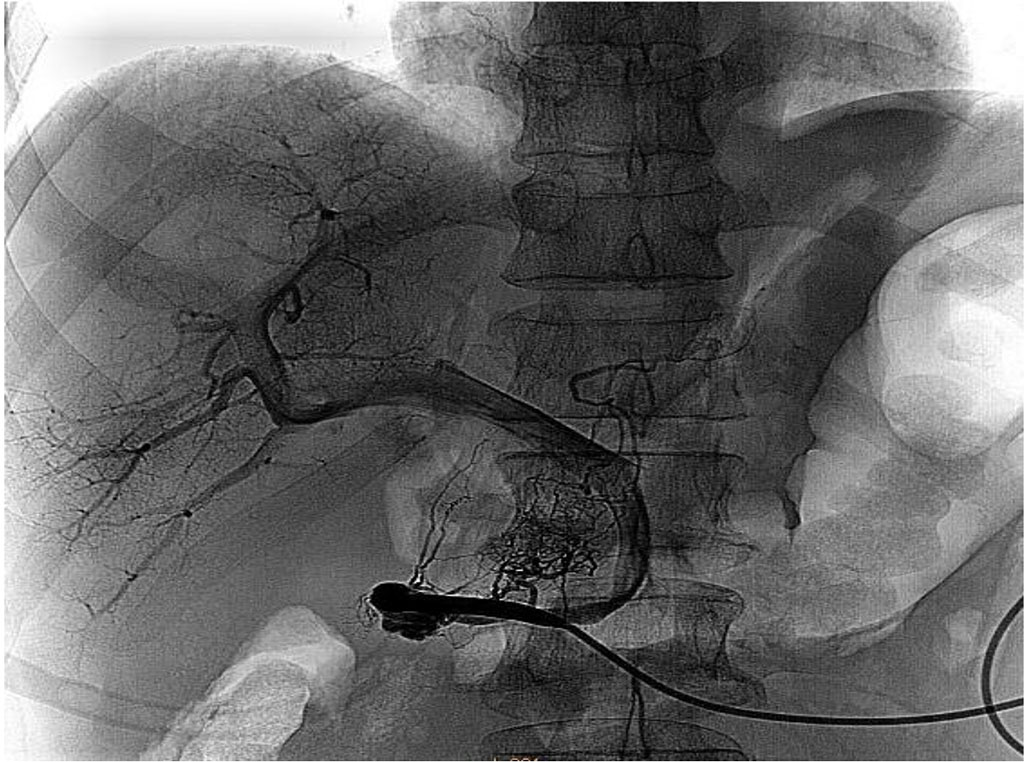
Figure 2 Control portography. The architecture of the portal system is visualized.
The end of the catheter with a pavilion and a plug and the end of the thread with the Roeder’s knot were output through a separate puncture to the left of the laparotomy wound. The catheter was fixed by suturing to the skin. The wound was covered with an aseptic bandage. After this, the patient was transferred to the ward and had continuous regional infusion therapy for 7-10 days. At the moment of catheter removal, using an endoscopic knot pusher, the Roeder’s knot was simultaneously tightened, ensuring reliable hemostasis. The thread from the Roeder’s knot was intersected at the level of subcutaneous fat.
In previously proposed methods, the vein was exposed to the skin, which, in our opinion, increased the risk of infectious complications. This modification, subject to the availability of instruments, as well as certain endosurgery skills, allows catheterization to be performed using a completely endoscopic approach. EIT was carried out by introducing drugs through the catheter: meglumine sodium succinate 10 ml/kg/day, ciprofloxacin 400 mg/day and ademetionine 400 mg/day for 3-5 days at a rate of 60 drops per minute. There were no complications after catheterization and EIT [20].
A total of 48 patients were treated. They were divided into two equal comparable groups – the main group and the comparison group. The research period was 2019-2022. The inclusion criterion for this study was the presence of complicated purulent peritonitis (Organ/Space Surgical Site Infection (OSSSI) and acute intestinal failure. All complications arose during the inpatient stage of treatment within a period of 1 to 23 days. Exclusion criteria were cases accompanied by septic shock, as well as the lack of technical capabilities for catheterization (if the gastrocolic ligament, the catheterization zone, was involved in the purulent process). The age of patients in the groups ranged from 19 to 82 years. In the main group, treatment was supplemented with EIT. In the comparison group, treatment was carried out according to the standard technique. Tab. 1.
Table 1 Stratification of patients in the study groups according to the nature and severity of complications
Complication |
Main group (n=24) | Comparison group (n=24) | ||
| Quantity | Clavien Dindo severity level | Quantity | Clavien Dindo severity level | |
| Liver abscess | 4 | IIIB-2; IVA-1; IVB-1 | 5 | IIIB-4; IVA-1; |
| Intra-abdominal abscesses | 6 | IIIB-4; IVA-2 | 5 | IIIB-1; IVA-4 |
| Perforation of acute gastrointestinal ulcers | 4 | IVB-4 | 4 | IVB-3; V-1 |
| Suture failure | 4 | IVA-2; IVB-2 | 3 | IIIB-1; IVA-1; IVB-1 |
| Intestinal fistulas | 2 | IVB-2 | 3 | IVB-2; V-1 |
| Recurrent peritonitis | 2 | IVB-1; V-1 | 2 | IVB-1; V-1 |
| Pancreatogenic phlegmon of the retroperitoneum | 2 | IVB-2 | 2 | IVB-1; V-1 |
To study enteral insufficiency, the enteral morphofunctional coefficient (EMFC) was dynamically calculated. Also, using the enzyme immunoassay method and the Cobas e411 analyzer (Switzerland), we determined total alkaline phosphatase (ALP), its intestinal isoform - intestinal alkaline phosphatase (IAP), as well as their ALP/IAP ratio (%) in blood serum and intestinal contents. Phagocytic activity of blood was determined by latex phagocytosis by neutrophils. The following indicators were studied: phagocytic number (PN), phagocytosis percent (PP) and number of active phagocytes (NAP). The severity of the patients' condition was assessed according to APACHE II (Acute Physiology and Chronic Health Evaluation), as well as according to V.S. Savelyev abdominal cavity index (ACI). The above indicators were studied on the day 1, 3, 5 and 7 of treatment.
Statistical relationships between indicators were assessed using the correlation module “Basic Statistics and Tables STATISTICA 10.0”. We used the adaptive randomization method. In order to determine the significance of p differences between groups, Student's t test and one-way analysis of variance with calculation of Fisher's F test were used. The reliability of data differences in the groups was assessed using the Mann-Whitney U-test for paired comparisons. Differences were considered statistically significant at p≤0.05.
When comparing the effectiveness of treatment on the scales of the severity of patients' condition (APACHE II, ACI), it turned out that statistically significant normalization of indicators occurred earlier in the main group, on average on the day 5. At the same time, in the comparison group, these changes occurred only on the day 7 of treatment (p≤0.05).
Intestinal failure was relieved earlier in the main group, which was confirmed by changes in EMFC. On the day 5, in the main group, it was 4.3±0.3, while in the comparison group, even on the day 7, it did not reach normal values and was 7.8±1.1 (p≤0.05).
Indicators of phagocytic activity of the blood increased statistically significantly in the main group by the day 3 of phage therapy, which was accompanied by an increase in PP and NAP. Tab. 2.
Table 2 Treatment results in the study groups (М±m)
Indicator |
Study groups (n=48) | |||||||
| Main group (n=24) | Comparison group (n=24) | |||||||
| Day 1 | Day 3 | Day 5 | Day 7 | Day 1 | Day 3 | Day 5 | Day 7 | |
| APACHE II | 21,3±0,4 | 16,3±0,3 | 9,5±0,3* | 10,7±0,3* | 21,6±0,2 | 17,3±0,3 | 16,9±0,4 | 12,7±0,3* |
| ACI | 19,3±0,5 | 15,8±0,4 | 11,6±0,5* | 10,7±0,4* | 19,1±0,5 | 17,9±0,4 | 16,6±0,3 | 12,7±0,3* |
| EMFC | 25,8±2,6 | 13,3±1,7 | 4,3±0,3* | 3,3±0,2* | 25,4±1,6 | 21,2±2,2 | 16,7±2,1 | 8,8±0,9* |
| ALP/IAP blood (%) | 0,71±0,14 | 0,62±0,06 | 0,42±0,08* | 0,43±0,11* | 0,82±0,07 | 1,34±0,09* | 0,92±0,11 | 0,88±0,13 |
| ALP/IAP chyme (%) | 6,21±0,16 | 3,17±0,11* | 3,22±0,08* | 3,11±0,09* | 6,11±0,14 | 8,65±0,14 | 7,99±0,15 | 6,55±0,21 |
| PP (N 65-95%) | 62,4±3,2 | 71,2±4,4* | 89,2±5,4* | 94,3±6,8* | 61,8±4,4 | 63,2±3,7 | 64,5±6,6 | 66,3±7,1 |
| PN (N 4,0-10,0) | 3,5±0,4 | 4,3±0,3 | 8,8±0,7* | 9,7±1,2* | 3,3±0,3 | 3,4±0,3 | 3,7±0,3 | 3,9±0,1 |
| NAP (N 2.5–2.9 thousand per 1 mm3.) | 1,8±0,3 | 2,7±0,2* | 2,7±0,3* | 3,1±0,2* | 1,5±0,5 | 1,9±0,4 | 2,1±0,3 | 2,4±0,1 |
| Average number of surgeries per patient | 2,3 |
4,5 |
||||||
| Bed days | 13,7 | 22,5 | ||||||
| Mortality | 1 | 3 | ||||||
Note: *-values at p≤0.05
In the comparison group, patients needed twice as many surgical interventions. There was a decrease in the length of hospital treatment in the main group by 8.8 days. Mortality in the comparison group was 3 times higher than in the main group.
Since the intestinal barrier is one of the most important components maintaining homeostasis in the gastrointestinal tract, loss of its integrity due to changes in bacterial composition, decreased expression of tight junction proteins and increased concentrations of proinflammatory cytokines can lead to intestinal hyperpermeability with subsequent development of other complications. Translocation of microorganisms and their toxic metabolites outside the gastrointestinal tract is one of the consequences of acute intestinal failure syndrome. Under normal conditions, the gut microbiome is isolated from the internal environment by the intestinal barrier. However, in some cases, dysfunction of the intestinal barrier integrity may allow harmful microorganisms, their antigens and their toxic metabolites to cross the intestinal barrier and enter first the mesenteric lymph nodes and then, through the portal system with the systemic circulation, into normally sterile host tissues and organs including the liver, lungs or brain [21, 22].
It should be noted that the use of EIT requires further study. It is necessary to clearly define the indications and contraindications for this technology, the timing of implementation, the composition of the medicinal “cocktail”, and evaluate it in the framework of “complexity - effectiveness”. Larger-scale studies will allow us to answer all those questions.
The proposed EIT technology can serve as a good supplement in treatment of patients with intra-abdominal purulent-inflammatory diseases complicated by acute intestinal failure. It prevents pathogenic bacterial translocation into the portal system and thus reduces the risk of developing generalized infection and multiple organ failure.
The authors have no conflict of interests to declare.