- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Cite as: Archiv EuroMedica. 2024. 13; 1: e1. DOI 10.35630/2024/14/1.103
Introduction: Laparoscopy has been used as an aid in the placement of abdominal ventriculoperitoneal (VP) shunts since 1993. Laparoscopic procedures are safe and allow direct visualization of the correct position of the catheter, and confirmation of shunt function.
Aim: The aim of this study is to describe our own method of introduction of the distal catheter in the right subdiaphragmatic space using the falciform ligament, which prevents catheter migration, distal obstruction and internal organ damage and compare it with existing literature.
Materials and Methods: Data was collected retrospectively for all VP shunt implantations and revisions performed in adults with hydrocephalus during the period November 2011 to November 2020. In all 51 cases of VP shunt insertion, the distal shunt catheter was implanted using our own modification of the laparoscopic method.
Results: In total, there were 66 shunt procedures performed during the study period. The majority of procedures (86.3% 57/66) were implantation of a new VP shunt, and 13.6% (9/66) were revision surgeries for replacement of a VP shunt due to catheter occlusion. Median follow up was 2.5 years. Most patients (89.5%, 51/57) underwent laparoscopic surgery.
Conclusions: Our modified laparoscopic method shows that this accessible, minimally invasive procedure is beneficial for patients with hydrocephalus and significantly decreases the rate of complications. The laparoscopic falciform technique significantly reduces the rate of distal VP shunt obstruction. The duration of surgery and the risk of infection have been also reduced. In addition, collaboration and simultaneous work of neurosurgeons and general surgeons strengthens companionship between departments.
Keywords: ventriculoperitoneal shunt, hydrocephalus, laparoscopic placement, falciform ligament, preventing obstruction.
The treatment of hydrocephalus with a ventriculoperitoneal (VP) shunt aims to provide drainage of the cerebrospinal fluid (CSF) into the designated peritoneal cavity to prevent increased intracranial pressure. Hydrocephalus may be congenital or caused by tumor, craniosynostosis, aqueductal stenosis, Dandy-Walker syndrome or idiopathic intracranial hypertension. The most common route of drainage of CSF is into the peritoneal cavity with other less frequently used drainage routes including ventriculoatrial, ventriculopleural, and lumbarperitoneal. Typically, the procedure is performed in a neurosurgery department, where a neurosurgeon independently implants the peritoneal catheter through an open minilaparotomy. Unfortunately, during minilaparotomy it is not possible to directly visualize the intraperitoneal catheter position and function of the shunt which can result in complications. To improve surgical technique and long-term effectiveness of VP shunts for the treatment of hydrocephalus, collaboration of neurosurgeons with general surgeons resulted in the introduction of laparoscopic techniques into the procedure. Laparoscopy has been used to aid in the placement of abdominal ventriculoperitoneal (VP) shunts since 1993 1-3.
Laparoscopy as a procedure is safe and allows direct visualization of the catheter position, and confirmation of shunt function. Research also shows that laparoscopic techniques result in fewer complications related to the procedure compared with minilaparotomy. For example, laparoscopy minimizes the occurrence of distal obstruction of ventriculoperitoneal shunt. In this paper we describe our own experiences of using a modified laparoscopic method of insertion and anchorage of the distal catheter in the right subdiaphragmatic space using the falciform ligament. This technique appears to reduce the risk of catheter migration, distal obstruction and injury to internal organs.
The aim of our study is to describe our own method of introduction and maintenance of the distal catheter of a VP shunt in the right subdiaphragmatic space using the falciform ligament as an anchorage point, preventing catheter migration, distal obstruction and damage to internal organ. In this study we have also compared our results with available literature. Our method may be particularly useful in patients with previous abdominal surgeries or shunt revisions or in obese patients.
We collected data retrospectively for all implantations and revisions of VP shunts in adults with hydrocephalus in the period from November 2011 to November 2020. There were 57 VP shunt implantations in total performed in our department utilizing cooperation with a general surgeon and laparoscopic implantation of the distal catheter. Out of these 57 shunt implantations, 51 were implanted laparoscopically, using our own modification of the laparoscopic method 4. In each procedure, the neurosurgeon and general surgeon performed their part of the surgery simultaneously, which resulted in reduction of operative time by approximately 35 minutes. Each patient gave signed consent for the procedure after reviewing the treatment plan and risk of possible complications. All patients consulted with a general surgeon the day before surgery to investigate and detect abdominal scars from previous operations and rule out other relevant pathologies.
Each patient was placed in a supine position following induction of general anesthesia, with the head turned slightly to the left (Figure 1). In each case, the neurosurgeon and general surgeon commenced their part of the procedure simultaneously. Access to the abdomen was gained at Palmer’s point, located 3cm below the left costal arch in the midclavicular line (Figure 2). Pneumoperitoneum was established using CO2 to a pressure of 12mmHg using a Veress needle (120mm or 150mm) through the incision site. After removal of the Veress needle, a 5.5mm metal trocar was inserted perpendicular to the abdominal wall using a conical or triangular corset. A 5mm laparoscope with an oblique optic at an angle of 30 degrees (Olympus 4K) was inserted through the same port. Exploratory laparoscopy was performed as standard, starting from the insertion point of the Veress needle and trocar, to ensure no iatrogenic damage of the abdominal organs had occurred. The peritoneal cavity was then inspected according to usual exploratory laparoscopy standards, starting from the right lower abdomen, clockwise through the epigastric region, to the rectosigmoid flexure. The aim of this procedure is to exclude possible pathology in the abdominal cavity, which may not have been apparent prior to surgery.
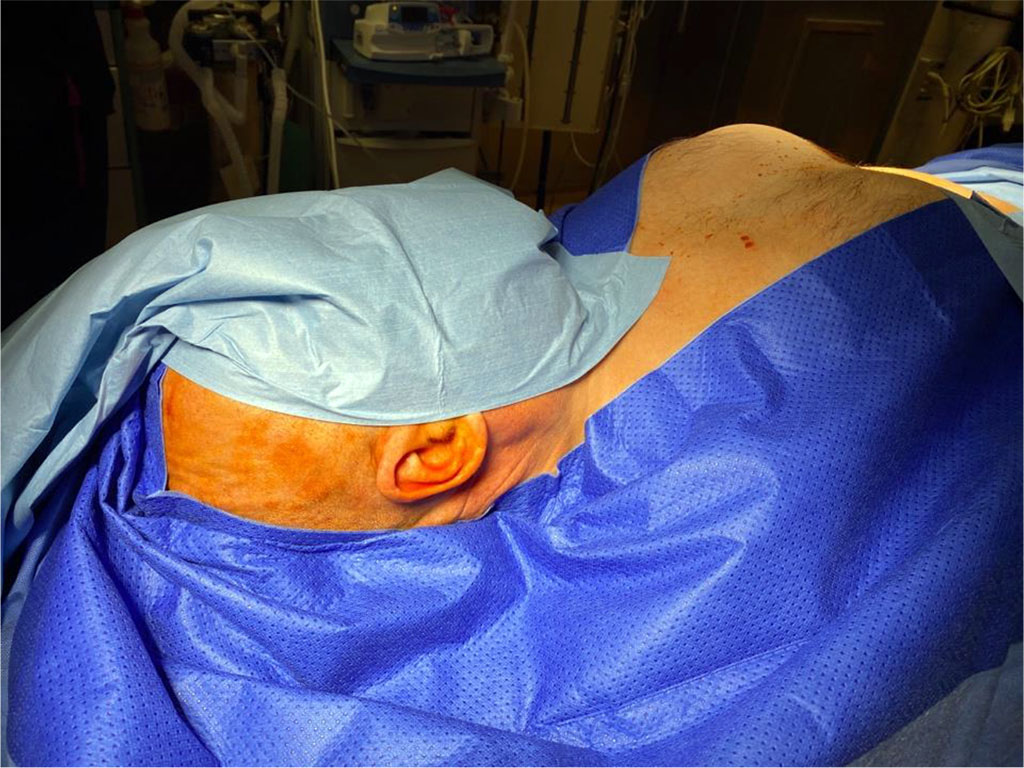
Figure 1 The patient supine positioning after induction of general anesthesia, with the head turned slightly. /Source: Author Content/
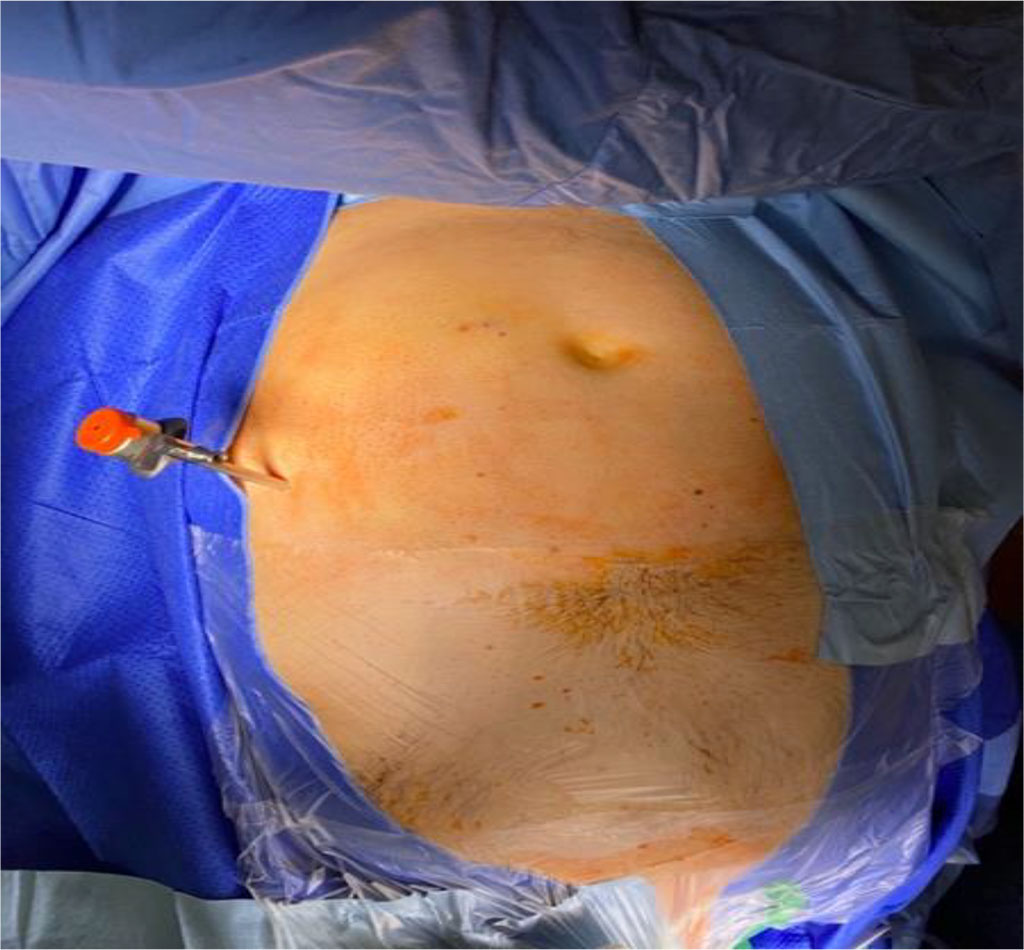
Figure 2 Access to pneumoperitoneum at the Palmer's point with a Veress needle (3 cm below the left costal arch in the mid-clavicular line). /Source: Author Content/
At the same time, the neurosurgeon makes a typical 3cm straight skin incision and a burr hole in the right frontal region of the skull at Kocher’s point. The anterior horn of the lateral ventricle is accessed, and the ventricular catheter inserted. The catheter is then connected via a one-way valve to the distal catheter. The catheter is tunneled subcutaneously to the retroauricular point where a 5mm skin incision is made. The catheter is then tunneled subcutaneously down the anterior aspect of the neck to the chest where it is continued 5–6cm inferolateral from the xiphoid process to another 5mm incision on the abdominal wall just below the left costal margin. The catheter is then held using dissector arms and inserted through the abdominal wall into the peritoneal cavity under direct vision using the camera (Figure 3).
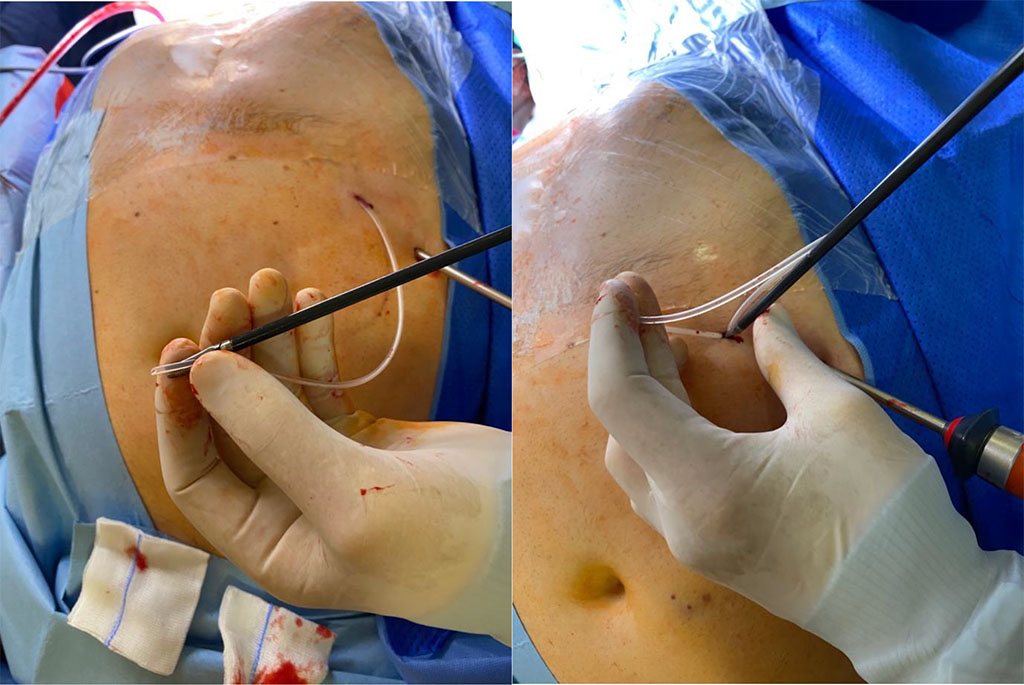
Figure 3 The drain was placed in the dissector arms and inserted through the abdominal wall into the peritoneal cavity under camera control in direct visualization. /Source: Author Content/
A 1mm hole is made in the falciform ligament of the liver (Figure 4) using a monopolar diathermy dissector. The distal catheter drain is fed through this and placed on the diaphragmatic surface of the right lobe of the liver (Figure 5). The length of the cannula, measured from the opening of the falciform ligament, is measured at 20 cm, which is sufficient to locate it above the liver. After the shunt is placed, a “drop test” is performed to visualize outflow of cerebrospinal fluid from the peritoneal tip into the abdominal cavity to confirm that the implanted system is working properly (Figure 6). The scalp and retroauricular incisions are sutured by the neurosurgeon and the trocar is removed from the peritoneal cavity under direct vision by the surgeon. The insertion port and cannula insertion point are closed using Algower self-adaptive absorbable subcuticular stitches. The abdomen is desufflated with no need for indwelling peritoneal drainage.
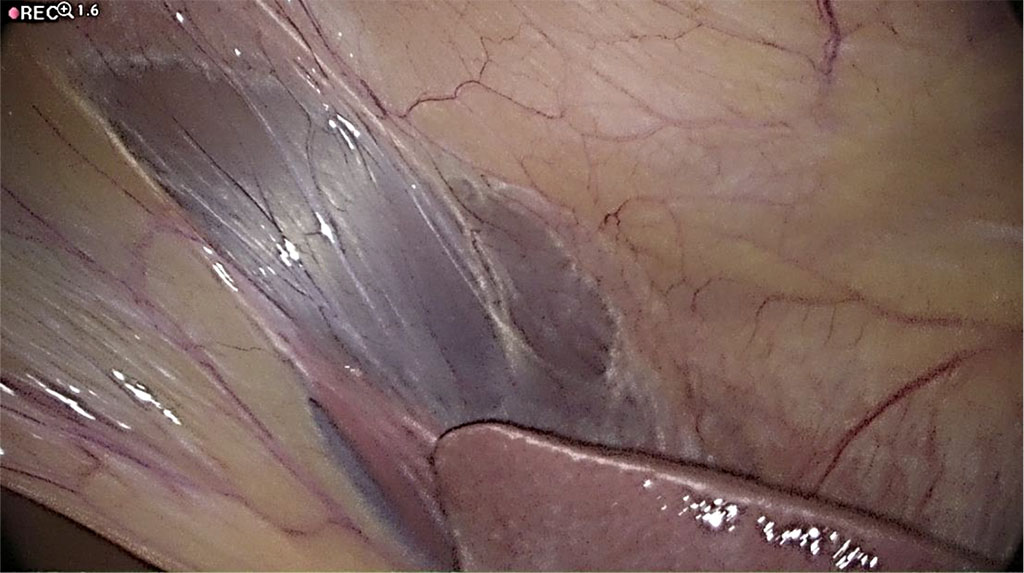
Figure 4 The falciform ligament of the liver. /Source: Author Content/
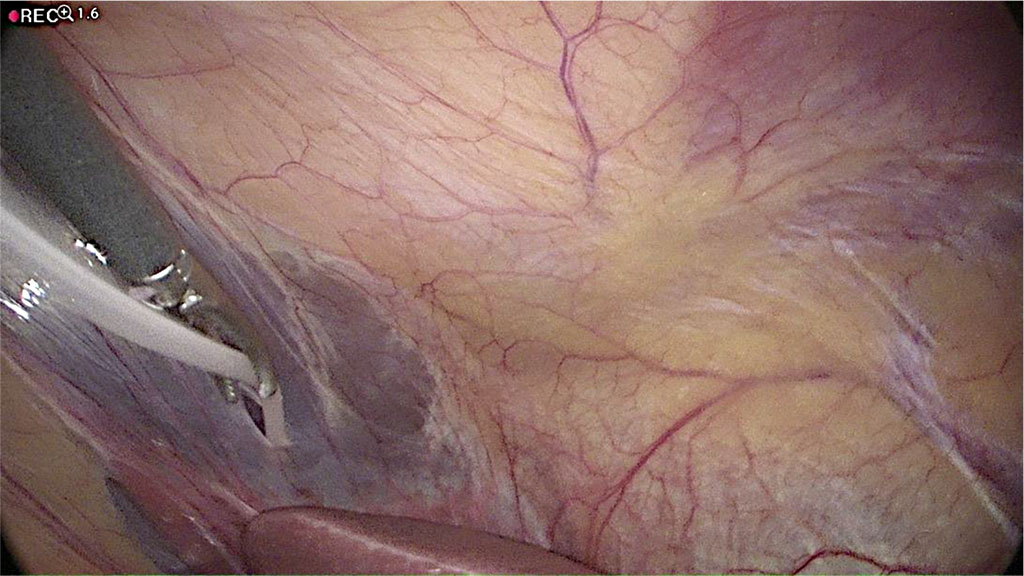
Figure 5 Catheter is passing through the hole in the falciform ligament. /Source: Author Content/
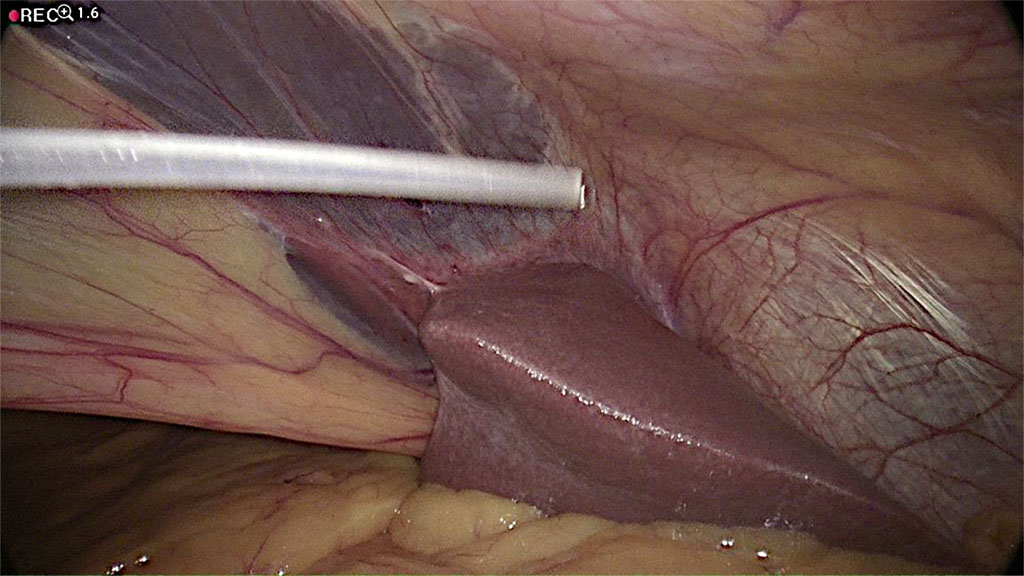
Figure 6 Performing of “drop test”- visualization of fluid out flow from distal peritoneal catheter intra-abdominally. /Source: Author Content/
In total, during the study period, 66 shunt procedures were performed. The majority (86.3% 57/66) was insertion of a new VP shunt, and 13.6% (9/66) were revision and replacement due to occlusion of the catheter. The ratio of female to male ratio was 1:1.6 and the mean age was 64.6 ± 18.2 years). Median follow-up was 2.5 years. 89.5% (51/57) of patients underwent laparoscopic surgery using our technique. All revision surgeries were due to occlusion of the catheter and followed earlier implantation using the minilaparotomy technique by a neurosurgeon. Five patients (7.5%) had proximal catheter obstruction requiring shunt revision. Two patients (3%) were found to have distal obstruction and 2 shunts (3%) did not work properly. None (0%) of the 51 patients who underwent the laparoscopic procedure were found to have evidence of catheter obstruction at the end of the study at the last follow-up visit.
Accurate determination of placement, position and movement of the distal catheter is of utmost importance for the neurosurgeon. Traditionally, the neurosurgeon uses an open minilaparotomy technique for placement of the shunt and insertion is performed by blind catheter insertion into the abdominal cavity. Some neurosurgeons prefer to insert shunts themselves, however, in hospitals where collaboration between departments is possible, partnership between the surgery and neurosurgery teams is welcome. Moreover, the interaction between the teams in the operating theatre builds good relations and increases collegiality.
The introduction of the above described technique prevents the most common complications that may occur as a result of implantation of the distal part of the shunt [5-7] . These complications are: dislocation of the peritoneal catheter, shunt malfunction (partial or complete blockage of the shunt), shunt infection, duration of the procedure, injury to intra-abdominal organs.
Positioning of the distal VP shunt in the peritoneal cavity can be problematic. The catheter may not be optimally positioned in the preferred area, and the catheter may become wrapped or engulfed within the peritoneum or omentum. Using the method we have described above, we prevent catheter migration as the catheter is located over the liver and is securely held by the falciform ligament. The cause of migration is unknown. Movements of the head and creation of positive pressure in the abdomen potentially cause movement of the catheter [8-10]. Nagasaka et al. [11] have reported that changing from a lying to a standing position can pull the catheter out from the peritoneal cavity. These authors [11] also reported that anchoring e.g. with a fibrous encasement prevents unpredictable movements of the catheter, and confirm the use of falciform ligament as an anchor.
There are many studies which established that the placement of the VP shunt via a laparoscopic approach influences the occurrence of distal catheter complications. He et al [12] performed a meta-analysis and reported that using a laparoscopic technique, rates of distal shunt failure were significantly reduced, with distal shunt failure occurring in 0%–15.7% of laparoscopically inserted peritoneal catheters and in 6%–28.7% of catheters inserted using a minilaparotomy technique. The authors also state that such results may be due to the insertion of the catheter being under direct camera control and therefore, during the procedure, adhesiolysis can be avoided. This may reduce the risk of formation of peritoneal adhesions, which can cause catheter obstruction [12]. However, when analyzing overall shunt failure (both distal and proximal), there was no significant difference in rates of shunt complications (laparoscopy = 17.5% vs. minilaparotomy = 20.7%; p = 0.53). Schucht et al. [13] published similar results, reporting that the overall rate of shunt complications was 15% in the laparoscopic group and 18.3% in the minilaparotomy group. Naftel et al. [14] also reported no difference between minilaparotomy and laparoscopic procedures in terms of overall rate of shunt complications, however peritoneal dislocation was found significantly less often in the laparoscopic group.
In our study, in the laparoscopic group, we had no cases of catheter dislocation. Bani et al. and Schucht et al. obtained similar results [13, 15]. Roth et al. [16] reported that in their open minilaparotomy group there were more cases of distal shunt obstruction than in the laparoscopic group (10.3% vs. 4%). Schubert et al. [17] reported similar results (12% vs. 4%).
Laparoscopic distal shunt placement prevents malfunction of the distal catheter due to the ability to directly visualize the function of the shunt via the camera. Use of the camera allows the ability to check the patency of the drain shunt using a “drop test”, which gives the surgeon confidence that the drain is not bent, broken or clogged with peritoneal tissue. The laparoscopic approach therefore is of benefit to the patient as it provides a guarantee that the shunt is performing correctly following insertion. There have been different laparoscopic techniques described regarding insertion of the distal catheter of shunt. In early reports, a three trocars technique was used with good results [1,2]. A peel-away introducer sheath has also been used to insert the catheter through the abdominal wall [18]. Use of a single trocar technique has also been described with equal success [4, 19-21]. The method we describe is using a single trocar, however the insertion location is different. The use of Palmer’s point as the insertion location reduces the likelihood of scars being present from previous surgical procedures and does not interfere with the course of future laparoscopic operations (i.e. opening of the scar). The use of Palmer’s point allows the catheter to be positioned accurately according to the operator’s plan.
Shunt infections are a common cause for shunt failure. Faillace et al. [22] found that in most cases, symptoms of infection occur around 2 months after the surgical procedure. The authors suggest that bacterial infection must occur during shunt placement [22]. Other published studies have reported that laparoscopic peritoneal catheter surgery can result in infection rates of 1% to 13.5% [15, 23-26]. He at al. [12] in their meta-analysis reported that there was no significant difference in overall infection rate between the minilaparotomy method (0%–1.3%) and the laparoscopic method (0%–2.7%). Naftel et al. [14] reported higher rates of infection in laparoscopic surgery compared with the minilaparotomy technique however this was not statistically significant (8.2% vs. 6.6%). Roth et al. [16] described a higher infection rate using the minilaparotomy method compared with the laparotomy method (13.5% vs. 7.2%). In the present study, there were no shunt infections in those implanted laparoscopically. Based on the available literature, using a laparoscopic method appears to be more favorable.
Shorter operative time is also important to reduce the incidence of infection. Bronitsky et al. [27] in 1993 stated that reducing the duration of surgery may markedly decrease the rate of postoperative complications. Later studies reported that longer surgery time results in a higher risk of shunt infection [28, 29]. A meta-analysis [12] reported the average operative time in a case series was 49–78 minutes for laparoscopically inserted peritoneal catheters and 116–120 minutes using the minilaparotomy method. The reduction in operative time can be attributed to the simultaneous operation of the abdomen by additional general surgeons using a laparoscopic method whilst the neurosurgeon accesses the ventricle [37]. Wang et al. [19] reported an average operative duration using a laparoscopic technique of 48 minutes (range 22–53 minutes). The reduction in operative time was potentially due to the use of a single trocar laparoscopic technique. This study also confirms the reduction in procedure duration using the laparoscopic technique described in this study. In our study, the duration of the entire VP shunt insertion procedure ranged from 26 to 51 minutes, with a mean surgery time of 34.6 minutes.
Injury of intra-abdominal organs may occur as an intraoperative complication. Using the minilaparotomy method, complications include small bowel injury during incision of the skin and peritoneal tissue, often associated with the presence of peritoneal adhesions. Using a laparoscopic approach, the surgeon can more easily avoid accidental injury to the small bowel, however laparoscopy is not free from complications. Establishing pneumoperitoneum can cause many of the potential intraoperative complications, with the majority of complications occurring during but not after establishment of pneumoperitoneum. Occurrence of complications during establishment of pneumoperitoneum is most often also associated with the presence of peritoneal adhesions and poor condition of the abdomen [16]. In our technique, we enter the abdomen at Palmer’s point, from which it is safer to perform pneumoperitoneum, particularly in patients who have undergone previous abdominal surgery or who are obese. Schucht et al. [13] reported that 1.7% of cases resulted in complications related to bowel lesions and in these cases, patients had undergone previous abdominal surgery. Argo et al. [31] also observed a higher risk of complications if there had been previous surgery performed on the abdomen. However, He et al [12] in their meta-analysis reported that there was no significant difference between surgical and laparoscopic methods in terms of intraoperative complications.
According to these publications, the placement of the peritoneal catheter may be more difficult in cases with a history of abdominal surgery, peritonitis, or patients who have previously undergone failed attempts at VP shunt insertion [3]. In these patients, the assumed location of the catheter may be different from the planned standard which may cause adhesions or malfunction of the shunt [16]. This, in turn, may necessitate further abdominal surgery which can lead to retropulsion of the incision, hernia or adhesions [32]. Therefore, using our modification and establishing access at Palmer’s point reduces the risk of interference with scars from previous surgical interventions and does not interfere with the course of future laparoscopic procedures and, thus, protects against such complications.
In current literature, the overall rate of shunt complications ranges from 12.9% to 46.3% [33-35]. Stone et al.[5] in a 15-year retrospective study concluded that 84.5% of patients required one or more shunt revisions. Naftel et al. [19] observed rates of shunt failure of up to 20.9%. Kestle et al. [36] reported that distal mechanical failure comprises 25–30% of all malfunctions. In our study, there were no distal catheter complications in any of the shunts implanted laparoscopically using our own technique during the follow-up period (median 2.5 years).
Our modified laparoscopic method is straightforward, minimally invasive and beneficial for patients with hydrocephalus requiring VP shunt placement, particularly for patients with obesity, adhesions or those requiring peritoneal shunt revision. The innovative approach used in the present study is adaptable, and can significantly reduce the risk of complications. The use of the falciform ligament to position and anchor the catheter significantly reduces the rate of distal VP shunt obstruction. The duration of surgery is reduced in comparison with open procedures, which decreases the risk of infection. In addition, the simultaneous operation by the neurosurgeon and general surgeons enhances cooperation between departments and strengthens companionships.
Authors have no conflict of interest to declare.
This publication was prepared without any external source of funding.
Mariusz Sowa - conceptualization, writing original draft, review and editing, project administration; Joanna Sowa – visualization, Wiesław Pesta – supervision.