- Home
- About the Journal
- Peer Review
- Editorial Board
- For Authors
- Reviewer Recognition
- Archive
- Contact
- Impressum
- EWG e.V.
Aspergillosis is mainly a lung infection in human. This is caused by the fungus Aspergillus. Though, most of the strains are harmless to humans, only a few of them can cause serious diseases mostly in immunocompromised individuals. The individuals with a medical history of respiratory problems are at high risk of infection. An aspergillosis patient if not treated well within time it may result in invasive aspergillosis developing lung fibrosis, huge hemoptysis and multiple organ failures, CNS and brain infections, endocarditis and death. The present paper deals with their disease causing abilities, diagnosis and treatment in human in the light of recent researches done so far in the field of medical microbiology and pathology.
Keywords: Aspergillosis, Aspergillus fumigatus, ABPA, CPA, Invasive aspergillosis, Death
This is quite surprising to learn that we, the people in 21st century still not quite aware and alert about the fungal infections even they are continuously killing us comprehensively. We are least bother about the fungal infections treating it very lightly. These fungal infections are killing more than a million of people with billions of causing new infections every year globally. Further, though these infections are more common in immunodeficient people, the other individuals who are otherwise healthy are also not exempted (Misch and Safdar 2016, Davda et al. 2018 and Kanj et al. 2018) [1-3].
Of all human fungal infections aspergillosis is one of them. This is caused by the cosmopolitan and most contaminating fungus Aspergillus initially described by a Roman clergyman and biologist Pier Antonio Micheli in 1729 (Bennett 1842) [4]. More than 300 species of the genus Aspergillus have so far been investigated (Samson et al.2014) [5]. The disease is developed in human when a sufficient number of spores are inhaled. It causes a kind of bronchopulmonary infection in which the lungs are primarily affected. The disease is called as the bronchopulmonary aspergillosis. Of the many species of Aspergillus, A. fumigatus and A. flavus are the two common species to develop pulmonary aspergillosis in human. Aspergillosis is frequently found in HIV/AIDS patients kept under the treatment of corticosteroids, organ transplantation, cancer patients and the patients hospitalized for long as nosocomial opportunistic infections (Denning et al. 2013 and Yun et al. 2017) [6,7].
Further, different types of aspergillosis have different symptoms. Despite pulmonary aspergillosis, other types of aspergillosis as cutaneous, nail, ear canal, nasal and sinus infections have also been reported in the human body. While some of them are mild, others are very serious (Romett and Newmann 1982, Van Burik et al. 1998, Mishra and Mehta 2004, Glass and Amedee 2011and Bongomin et al. 2018) [8-12].
The present paper is an attempt to review the status of current researches done so far in the field of aspergillosis.
Aspergillosis has been reported from all over the world. Nearly, I to 4 million peoples are affected from allergic bronchopulmonary aspergillosis (ABPA) globally. It can affect the individuals of any age. According to an estimate about 10% of the individuals suffering from cystic fibrosis or asthma experience to an allergic reaction of Aspergillus. Similarly, the individuals undergone organ transplantation with immunocompromization therapies are facing 50% more chances of developing invasive aspergillosis and high mortality rate in future (Stevens et al. 2003, Denning et al. 2013, Haider and Singh 2018) [6,13,14]. However, the exposure to Aspergillus has rarely caused problem for the people with healthy immune systems, this is usually not contagious from person to person (Denning 2010) [15].
Aspergillosis usually affected the lungs with the symptoms associated as fever, night sweats, a cough with sputum, fatigue, malaise and unexplained weight loss. The patients might also coughed up blood in sputum. Depending upon the severity of infections, the disease is broadly categorized as allergic, chronic and invasive aspergillosis grouped as under:
(Romett and Newman 1982, Bellini et al. 2003, Mishra and Mehta 2004 and Agarwal et al. 2013) [8, 10,16,17]
(Denning et al. 2003 and Lee et al. 2004 and Yun et al. 2017) [7, 18,19]
(Cornely et al. 2018) [20]
(Van Burik et al. 1998, Bongomin et al.2018 and Avkan et al. 2020) [9,12,21]
(Shelders et al. 2012, Helene et al. 2020) [22,23]
(Kieren et al. 2021) [24]
The infection caused by the Aspergillus initially developed the inflammation in lungs and sinuses causing ABPA and sinusitis respectively. In fact, both the conditions might be treated as the happenings prior to infection (Glass and Amedee 2011 and Agarwal et al. 2013) [11,17]. Similarly, while in chronic pulmonary aspergillosis, the disease is progressed as coughs with blood, the life- threatening invasive aspergillosis took place when these infections are disseminated in other body parts damaging various organs and systems of the body (Mahedano et al. 2018)[25].
The pulmonary aspergillosis infection is caused mainly by the Aspergillus fumigatus and sinuses and ear canal infections are caused by the A. flavus and A. niger respectively. Moreover, nearly 75% cases of otomycosis is caused by the A.niger (Denning et al.2003,Lee et al.2004 Glass and Amedee 2011, Agarwal et al.2013 and Yun et al. 2017)[7,11,17-19]. Cutaneous aspergillosis occurs through a break in the skin via postsurgical infections and trauma as wound infection. This is generally caused by a variety of Aspergillus species including A. flavus, A. fumigatus, A. niger and A. terreus(Van Burik et el. 1998 and Avkan et al.2020)[9,21].Approximately, 35% of all cases of onichomycosis are due to the infection of Aspergillus. A flavus, A. niger and A. terreus have been the most common etiological agents reported for onichomycosis in human (Bongomin et al.2018) [12].
Further, the use of azole fungicides for the plant protection and the use of triazole antifungal drugs in human has developed the azole – resistant A. fumigatus infections. Individuals are continuously being infected with the azole – resistant strains of A. fumigatus developing resistant invasive aspergillosis in human. In fact, these patients are more likely to die with azole – resistant A. fumigatus infections (Shelders et al.2012 and Helene et al.2021) [22,23].
There are very limited data available about the incidence of aspergillosis in patients suffering from cancer. However, an increasing trend of aspergillosis has been observed in cancer patients (Chien et al. 2022) [26]. Sometimes, the invasive pulmonary aspergillosis (IPA) is found mimicking as the metastatic lung cancer (Michiel et al. 2018) [27]. Lastly, the patients suffering from respiratory infections like COVID-19, influenza or pneumonia are more prone to develop lung aspergillosis (Amir et al. 2020, Bongomin et al. 2020, Frederike et al. 2020, Koehler et al. 2020 and Florian et al.2021) [28-32].
As the tiny spores of this fungus are unavoidable, normally found in outdoor and indoor environments they cause initially an allergic bronchopulmonary infection (ABPA) in human giving rise to the symptoms as headache, fever, chills, rhinitis, wheezing and coughs accompanied by the stuffy nose following drainage that may contain blood. However, this is yet to understand exactly that why the allergic reactions occur. Similarly, the mucus produced in the lung airways also provided a food environment for the mold to grow. Recurrent allergies may cause bronchiectasis characterized by the scarring of the lung tissues and widening of central airways. If not treated timely, the ABPA may show more severe symptoms with the chronic inflammations of the respiratory tract as bronchitis, asthama, shortness of breath, chest and Joint pain and rashes on body. Similarly, chronic pulmonary aspergillosis (CPA) patients, in addition to the symptoms mentioned above may also reveal the symptoms as cystic fibrosis, hemoptysis with permanent breathing problems. Finally, quite worsening the disease progression, invasive aspergillosis may occur where the infection reaches to other parts of the body as well. Most specifically it occurs in blood vessels. Despite all efforts the absolute mortality rate of invasive aspergillosis patients are achieved (Kosmidis and Denning 2015 and Mahedano et al 2018) [25,33].
The disease is diagnosed with the help of skin, blood and sputum tests and the culture and biopsy. Chest X-ray and CT/CAT scans are also carried on to visualize symptoms of the disease. Often the patients suffering from chronic pulmonary aspergillosis are seen with cavitation fungal balls or aspergilloma and nodules in their lungs (Horvath and Dummer 1996 and Misch and Safdar 2016) [1,34]. Further, the level of galactomannan is measured as a marker for aspergillosis. Galactomannan enzyme-linked immunosorbent assay (ELISA) is performed. Similarly, serum assays for D-glucan is also done. Aspergillus polymerase chain reaction (PCR) is also carried out for the diagnosis of aspergillosis in human (Stubbins and Warnock 1982, Horvath and Dummer 1996, Marty and Koo 2009, White et al. 2013&2015, Leeflang et al. 2015 and Misch and Safdar 2016) [1,34-39].
Aspergillus fumigatus accounts for almost 90% of the infections in humans. It has been the most common to cause pulmonary aspergillosis. (Marisa 2019)[40]. The main route of the entry of the same fungus in the human body is by the inhalation of spores to cause allergic bronchopulmonary aspergillosis (ABPA) developing severe asthma with fungal sensitization (SAFS).The incubation period varies depending on the dose of Aspergillus and the host immune responses. The peoples already suffering from chronic lung problems such as asthma, bronchitis, cystic fibrosis and immunocompromisation or certain pulmonary conditions like tuberculosis, emphysema or sarcoidosis are more prone to have the disease (Stevens et al. 2003, Agarwal et al. 2013, Denning et al. 2013 and Jack and Bajaj 2022)[6,13,17,41].If ABPA is not cured the chronic pulmonary aspergillosis (CPA) is developed. This is characterized by the hemoptysis and coughing with blood, chest pain, fever, shortness of breath and fatigue. And this is all due to the formation of fungus balls, aspergilloma or mycetoma produced by the fungus in air cavities of the lungs. It causes scarring or fibrosis of the lung tissues developing the lung cavities allowing the fungus balls to grow in them. One or more fungal balls may be present in the lungs. Aspergilloma is a tangled mass of fungal fibers, mucus, tissue debris, inflammatory cells, WBCs and the blood clotting protein fibrin. The people having more air cavities are in more risk of developing aspergilloma in their lungs. Therefore, CPA is sometimes more specifically called as the chronic cavitary pulmonary aspergillosis. But, in some cases of CPA, exceptionally Aspergillus nodules are also observed in lungs without the formation of cavities. These are the tiny masses without the formation of infected tissues (Denning et al. 2003 & 2013 and Yun et al. 2017) [6, 7,18].
Invasive aspergillosis occurs when an individual suffering from CPA is not cured properly. This is a most life- threatening and lethal condition in human in which the infections are spread in other parts of the body causing damage and failure of different organs including brain, heart, liver and kidneys. It occurs mostly in immunocompromised patients due to chemotherapy, bone marrow transplantation or suffering from other disorders (Mahedano et al. 2018) [25].
Further, invasive pulmonary aspergillosis in patients with liver failure or liver cirrhosis has always been fatal (Bbosa et al.2013 and Xuan et al.2018) [42,43].This is hypothesized that the fatality might be due to the result of toxins produced by the same fungus. Similarly, neuroaspergillosis developed brain infections having devastating consequences as brain abscess, meningitis, cerebritis, cranial sinus thrombosis and ventriculitis. It penetrated the blood – brain barrier to cause damage to the CNS(Marisa 2019)[40]. Aspergillus infection in human heart is rare appearing very difficult to treat as the high mortality rate is achieved. The infection when enters the blood stream it travelled to the heart. Invasive pulmonary aspergillosis (IPA) sometimes also appeared as a lethal complication after heart transplant and surgery. Nearly 20% of deaths occur in the first year after transplantation (Ameeta et al.2010, Beata et al.2019, Christina et al.2018 and Eduardo et al.2021) [44 - 47].
A kidney infection is a type of urinary tract infection (UTI) usually called as pyelonephritis. Although, Aspergillus pyelonephritis is rare but often observed in immunocompromised individuals. Kidney and bladder are more frequently involved in invasive aspergillosis. Nearly half the patients suffering from renal aspergillosis had renal failure (Sakamoto et al.1978, Godec et al.1989, Wise and Silver 1993 and Zhou et al.2017[48 – 51]. Similarly, Aspergillus infection in renal transplant (Maanes et al.1996, Hadaya et al.1998, Guleria et al. 1998, Shirwany et al. 1998, Sagorika et al.2013 and Antonio et al.2020) [52 – 57] and prostatic aspergillosis have also been reported (Karayannopoulos and stylianea 1981, Khawand et al. 1989, Campbell et al. 1992, Abbas et al. 1995, Hemal et al.1999 and Valerio et al. 2009) [58 – 63]. Lastly, the role of genetic and immune disregulation caused by the pathogenicity of aspergillosis is still a matter of further research (Bongomin et al. 2020) [29].
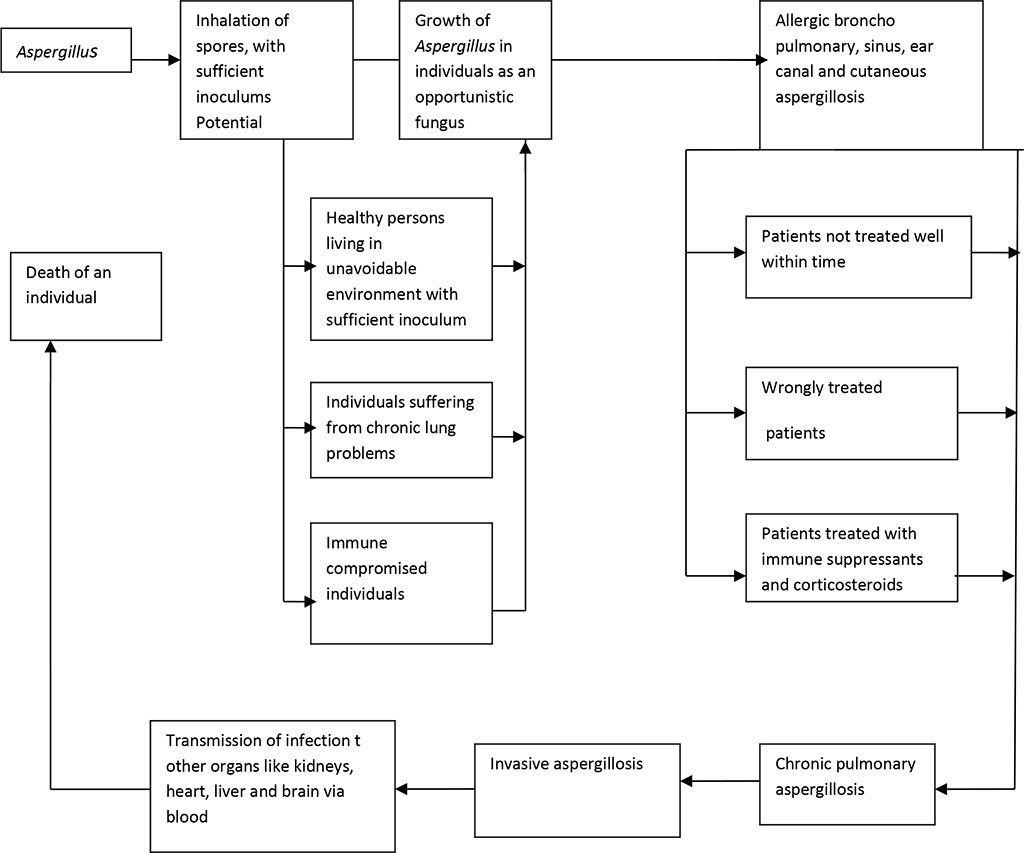
Figure 1. A functional model of developing aspergillosis in human
While the prognosis for patients with allergic pulmonary and sinus aspergillosis is good unless the lungs and sinuses are not severely damaged, this is quite poor with the invasive aspergillosis patients. If the infections are spread to other organs of the body especially CNS, heart, liver and kidneys, the mortality rate observed is very high (Alastruey et al. 2018) [64]. Sometimes, a very weird situation is also developed when a medical practitioner differentially misdiagnosed and treated a patient as a case of pulmonary tuberculosis.
The aspergillosis patients are treated with some antifungal drugs like variconazole, posaconazole, itraconazole and amphotericin B. Variconazole is the first choice of drug for physicians. Amphotericin B, though effective, usually not prescribed due to its adverse side effects. Sometimes, caspofungin is used for resistant strains of Aspergillus. Micafungin has also been tried. The most common drug of choice for physicians as corticosteroids are prednisone, prednisolon and methylprednisolon. As usual, allergic bronchopulmonary aspergillosis is being treated with itraconazole and corticosteroids (Jenks and Hoenigl 2018)[65].Moreover, physicians should always be acquainted that all antifungal drugs can have serious side effects including kidney and liver damage, if used for a longer period of time. Similarly, triazole antifungal drugs are the primary treatment for aspergillosis. However, the patients treated with azoles if developed azole resistant A. fumigatus are 33% more likely to die than the patients simply treated azoles (Shelders et al.2012 and Helene et al.2021) [22,23].
Further, the patients suffering from chronic pulmonary and invasive aspergillosis, the treatment should begin as early as possible. If not treated within time, lung fibrosis may occur. The treatment response is measured by the disappearance of fungus balls and decreasing the cavity lesions. Surgery with antifungal treatment is required for the removal of fungus balls or aspergilloma. The excessive bleeding caused by the aspergilloma can only be prevented by surgery. However, this is only recommended for advanced and chronic cases of aspergillosis as it does not work properly and the bleeding may recur again. It has also been observed that therapeutic embolization also does not work for long (Lang et al. 2020 and Fosses and Waymac 2022[66,67]. Similarly, on the off chance, surgery for the infected heart valves is also done to expel the tainted regions.
Finally, as the invasive aspergillosis is usually rapidly progressive, it eventually leads to death. Various specialists like allergists, thoracic surgeon and a radiologist are always required for the treatment of aspergillosis patients in association with a good physician.
Aspergillosis is a kind of bronchopulmonary infection caused by the fungi. The causal organism is Aspergillus. Initially, the Aspergillus fumigatus causes allergic bronchopulmonary aspergillosis (ABPA) in human. If not treated well within time, a rather more serious form of the disease developed. This is chronic pulmonary aspergillosis (CPA) damaging the lungs by developing aspergilloma. The disease is more easily developed in immunocompromised individuals. However, the healthy individuals are also not otherwise exempted from infection with the availability of sufficient inoculum potential. It has also been observed that the individuals suffering from different ailments and diseases developed the aspergillosis differently as under:
Finally, the sign and symptoms of aspergillosis may vary with the type of illnesses developed. CPA is often characterized by the rhinitis, coughs with blood and fever, chills, chest pain, night sweats, bronchitis, asthma and shortness of breath as dyspnea. The final stage of disease as invasive aspergillosis occurs when the infections are spread in other body parts via blood. The diagnosis for aspergillosis is often made with the help of sputum and blood test, thoracic x rays, CT scan, ELISA and PCR. The initial stage treatment is easy with certain antifungal medications and corticosteroids. The first choice of drug for physicians is itraconazole and corticosteroid as prednisolone. Sometimes, surgery is also required for the removal of aspergilloma. Lastly, the treatments should begin for aspergillosis as soon as possible because the survival rate for patients suffering from invasive aspergillosis is very low. Sometimes, the treatment with some antifungal medications should begin as soon as the aspergillosis is suspected, even before the diagnosis has confirmed the disease.
CNS Central nervous system
HIV Human immune virus
CPA Chronic pulmonary aspergillosis
PCR Polymerase chain reaction
WBC White blood corpuscles
IPA Invasive pulmonary aspergillosis
UTI Urinary tract infection
COPD Chronic obstructive pulmonary disease
AIDS Acquired immunodeficiency syndrome
ABPA Allergic bronchopulmonary aspergillosis
SAFS Severe asthma with fungal sensitization
ELISA Enzyme linked immunosorbent assay
CT scan Computed tomography scan
CAT scan Computed axial tomography scan
This piece of research work is dedicated to the memory of marhooma Shahidan Khatoon, mother in law of my niece. She was very much loving and caring to us. She has been suffering from chronic obstructive pulmonary disease (COPD) and died recently of lung failure.
The authors have declared no conflict of interest. They have approved the final version of the manuscript contributing equally.
No financial support was granted during the course of this research work.
As this is purely a review article therefore, it does not require any ethical clearance.